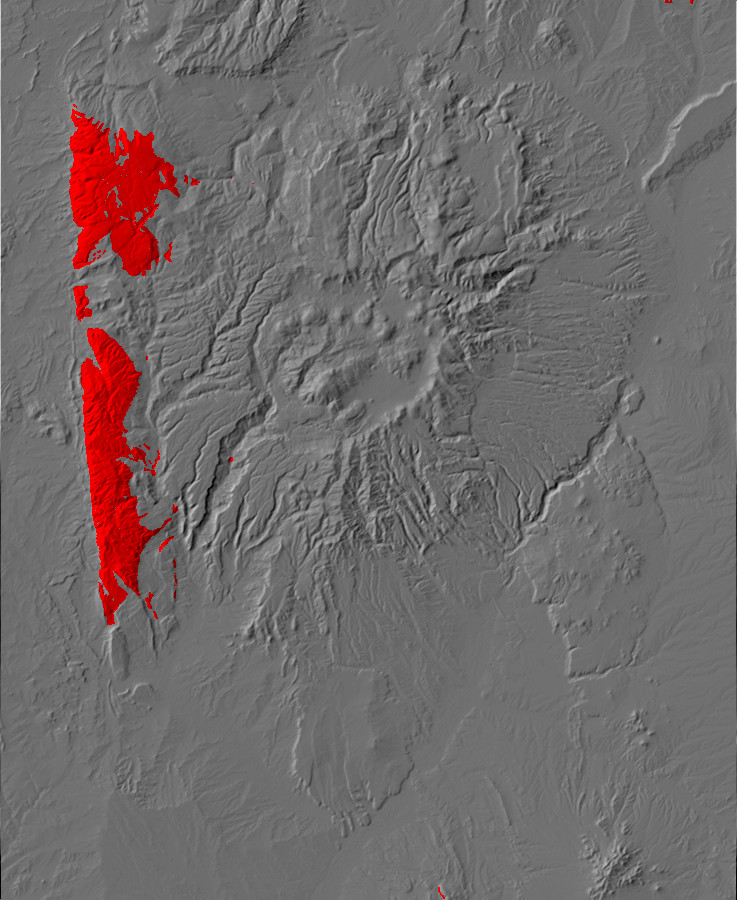
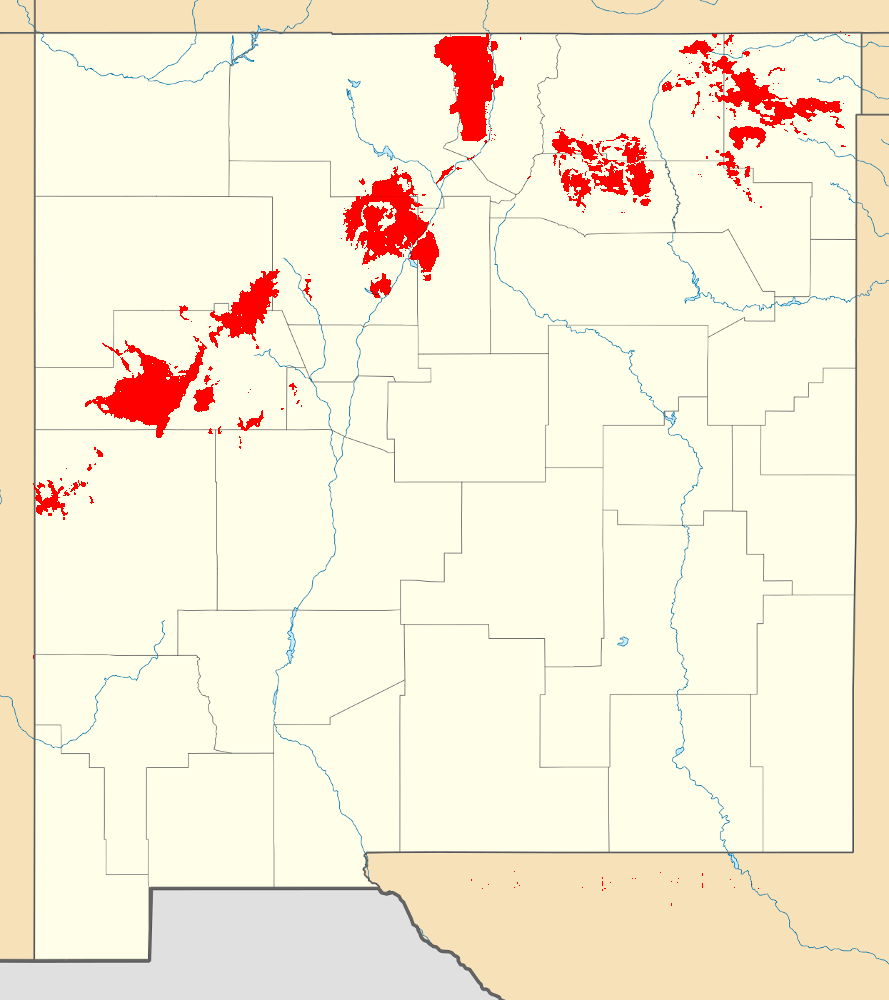
Relief map of the Jemez with Precambrian outcroppings highlighted in red.
Table of contents here.

The Earth of 1.8 billion years ago was a very different world
than the Earth of today. The atmosphere was thick with carbon
dioxide and had less than 10% of its current abundance of oxygen.
Though there were continents, they were wastelands barren of life,
and even the oceans contained only primitive microorganisms. It
was in this setting that northern New Mexico first came into
existence.
The first chapter of this book began the story of the Jemez
Mountains with the formation and early history of the Earth.
In this chapter, we will look at the oldest geologic features of
the Jemez area.
In the changes of the world the shapes of lands and of seas have been broken and remade; rivers have not kept their courses, neither have mountains remain steadfast; and to Cuiviénen there is no returning.
-- J.R.R. Tolkien, The Silmarillion

Relief map of the Jemez with Precambrian outcroppings
highlighted in red.
In the early days of scientific geology, geologists found that sedimentary
rocks (rocks formed from sediments eroded from older rocks)
often had distinctive collections of fossilized organisms in them.
In most locations, the lowest sedimentary rock beds contained
fossils of more primitive forms of life than the higher beds.
Geologists worked out a regular progression from the most
primitive fossils to fossils much like animals seen today. This
allowed sedimentary beds found at widely separated locations, but
containing similar fossils, to be correlated. Although the
absolute age of the rocks could not yet be determined, the
relative age could. Geologists worked out a time scale based on
relative age and began giving names to each interval of geologic
time. The three main eras in the fossil record were named the
Paleozoic ("ancient life"), the Mesozoic ("middle life"), and
Cenozoic ("new life"). Each era was further broken down into
periods, such as the Cambrian Period at the beginning of the
Paleozoic Era.
Geologists recognized that, in many places in the world, there were rock layers beneath the Cambrian Period beds that contained no fossils. These Precambrian rocks, as they are often still called today, are a mess. They tend to be coarsely crystalline rocks, either intrusive volcanic rocks or metamorphic rocks (rocks recrystallized under great heat and pressure.) They are often highly deformed and fractured. Precambrian sedimentary beds could not be correlated because they contained no fossils. Thus this Precambrian "basement" was all but indecipherable.
The discovery of radioactivity led to the invention of
radiometric dating of rocks in 1907 by American geologist Bertram
Boltwood. Geologists were finally able to assign absolute dates to
the various periods in the geologic record. They discovered that
the oldest Cambrian rocks are about 541 million years old, while
the earth itself, as we've seen, is about 4.55 billion years old.
In other words, the fossil-bearing sedimentary beds make up just
the last 12% of the Earth's history, and the Precambrian rocks
made up the other 88% of the Earth's history. With the ability to
determine ages for the Precambrian rocks, geologists could finally
started making sense of the Precambrian rock record.
Geologists now divide the geologic history of the Earth up into
four eons. These are the Hadean (> 4 billion years ago), the
Archean (4 to 2.5 billion years ago), the Proterozoic (2.5 billion
to 541 million years ago), and the Phanerozoic (541 million years
ago to the present.) Eons are divided into eras, which are
further divided into periods, which are divided into epochs. The
following table summarizes these divisions of time. You may find
it useful to bookmark this table for
easy reference as you read the rest of this book. Time before the
present in this table is given in units of ka, thousands of years,
and Ma, millions of years
| Eon |
Era |
Period |
Epoch |
|
|---|---|---|---|---|
| Phanerozoic (541 Ma
to present) |
||||
| Cenozoic (66.0 Ma to
present) |
The Age of Mammals |
|||
| Quaternary (2.58 Ma
to present) |
The Age of Man | |||
| Holocene (11.7 ka to present) |
Historical Man |
|||
| Pleistocene (2.58 Ma to 11.7
ka) |
||||
| Neogene (23.03 to
2.58 Ma) |
||||
| Pliocene (5.333 to 2.58 Ma) |
||||
| Miocene (23.03 to 5.333 Ma) |
||||
| Paleogene (66.0 to
23.03 Ma) |
||||
| Oligocene (33.9 to 23.03 Ma) |
||||
| Eocene (56.0 to 33.9 Ma) |
||||
| Paleocene (66.0 to 56.0 Ma) |
||||
| Mesozoic (251.9 to
66.0 Ma) |
The Age of the Dinosaurs |
|||
| Cretaceous (145.0 to
66.0 Ma) |
||||
| Jurassic (201.3 to
145.0 Ma) |
||||
| Triassic (251.9 to
201.3 Ma) |
||||
| Paleozoic (541 to
251.9 Ma) |
||||
| Permian (298.9 to
251.9 Ma) |
||||
| Pennsylvanian (323.2
to 298.9 Ma) |
||||
| Mississippian (358.9
to 323.2 Ma) |
||||
| Devonian (419.2 to
358.9 Ma) |
||||
| Silurian (443.8 to
419.2 Ma) |
||||
| Ordovician (485.4 to
443.8 Ma) |
||||
| Cambrian (541 to
485.4 Ma) |
||||
| Proterozoic (2500 to
541 Ma) |
||||
| Neoproterozoic (1000
to 560 Ma) |
||||
| Mesoproterozoic (1600
to 1000 Ma) |
||||
| Paleoproterozoic
(2500 to 1600 Ma) |
||||
| Archean (4000 to 2500
Ma) |
||||
| Hadean (4550 to 4000 Ma) | ||||
I have omitted epochs before the Cenozoic Era, periods before the Phanerozoic Eon, and eras before the Proterozoic Eon, since these are still being formally defined and will not be much referenced in this book.
Making sense of the Precambrian remains a challenge.
Geologists must use all their ingenuity to extract clues from the
ancient and tortured rock beds dating from before the start of the
Phanerozoic. However, we are beginning to have a coherent story to
tell about the beginnings of northern New Mexico.
1.8 billion years ago, during the late Paleoproterozoic, a vast and barren continent lay beneath a leaden sky. This was Laurentia, which would someday become the core of North America. The sun shone low, for the west coast of Laurentia, which would someday become southern Wyoming, lay north of 60 degrees latitude. Compared with its orientation today, Paleoproterozoic Laurasia was rotated clockwise by more than ninety degrees, so that the coastline facing west would face south today. Most of the rest of the Earth's continental crust lay to the south and east, where it had assembled into the supercontinent of Columbia. A broad stretch of ocean, studded with islands, lay to the west. The islands were part of a set of island arcs where new crust was forming from magma extracted from the underlying mantle.
The surface of the continent appeared devoid of life, for the
first true land plants would not appear for another 1.3 billion
years. However, life was already present in the coastal waters,
and had been since early in the Archean.
Archean life consisted only of bacteria and archaea, two of the three domains of life currently found on Earth. Both have very simple cells that lack true nuclei or other internal compartments. Oxygen was virtually absent from the Archean atmosphere. The Sun, having left behind its exuberant infancy, shone at only about 75% of its current brightness, but abundant atmospheric methane trapped enough heat to permit the oceans to remain unfrozen.
The earliest record of life on Earth may be deposits of graphite, the soft crystalline form of carbon used in pencils, in the Isua region of Greenland. These are around 3.8 billion years old. However, this remains a matter of debate, since abiogenic graphite can form from ferrous carbonate at high temperatures. The evidence that at least some of the graphite at Isua is derived from ancient life includes the carbon isotope ratio, 13C/12C, in the graphite. The enzymes of living organisms are selective enough that they react significantly more slowly with molecules containing the 13C isotope than the much more common 12C isotope, and, as a result, biogenic carbon is depleted in 13C compared with the cosmic ratio. Some of the graphite of the Isua region shows such depletion, and, under the electron microscope, the graphite particles are seen to take the form of tubes and granules rather than the flaky grains typical of abiogenic graphite.

Fossilized Precambrian stromatolites from Glacier
National Park. National
Park Service
The earliest widely accepted evidence of life dates to some 300 million years later and takes the form of stromatolites. These are distinctively layered rock mounds, around a meter (3') in size, produced by massive colonies of cyanobacteria. Cyanobacteria, formerly known as blue-green algae, are capable of producing oxygen by photosynthesis. Stromatolites have been found in Archean rocks that are 3.5 billion years old. Stromatolites became widespread during the Proterozoic Eon, then declined sharply, likely because other forms of life evolved that feed on the microorganisms making up the colonies. Today, living stromatolites are found only in unusually harsh marine environments in which predators cannot survive.

Oscillatoria, a common modern blue-green alga,
from the author's aquarium.
The oxygen generated by Archean cyanobacteria was removed from
the environment as fast as it was produced. It rapidly combined
chemically with reduced iron and sulfur dissolved in the ocean
water. We know that oxygen levels were very low during the
Archean, because Archean river beds preserved in the rock record
sometimes contain grains of pyrite or uraninite. Pyrite, FeS2,
"fool's gold", is a compound of ferrous iron with reduced sulfur,
and while it is common in volcanic rock and in sedimentary rock
deposited in low-oxygen conditions, it is unknown in modern river
deposits. It quickly oxidizes in today's high-oxygen atmosphere.
Uraninite, UO2, likewise rapidly oxidizes to U3O8
under modern conditions.
However, by the start of the Proterozoic Eon, life was beginning
to change the face of the earth, as oxygen produced by
cyanobacteria exhausted the supply of reduced iron and sulfur and
began to accumulate. This is known as the Great Oxygenation Event.
The accumulating oxygen produced two distinctive geologic
signatures, both showing that the ferrous iron (Fe+2)
of the young Earth was being oxidized to ferric iron (Fe+3).
Banded iron formation from Michigan. Wikimedia
Commons
One signature is banded iron formations. These are massive beds
of chert (fine-grained silica), magnetite (Fe3O4),
and hematite (Fe2O3) in thin
layers. Unlike ferrous iron, which is moderately soluble in water,
ferric iron is highly insoluble, and it precipitated out of the
oceans in large quantities to form the banded iron formations.
Banded iron formations are almost always Paleoproterozoic in age,
between 2.4 and 1.8 billion years old, and they are now the most
important source of iron ore. The oldest rocks found in New Mexico
are Proterozoic rocks about 1.77 billion years old, and banded
iron formation is found in New Mexico in the Tusas
Mountains.
The other signature of free oxygen is the presence of sedimentary
red beds, which derive their bright red color from hematite.
Evidence of Archean red beds has been found in the Timiskaming
district of eastern Ontario, but the first widespread occurrence
of red beds was around 1.8 billion years ago.
By around 2.3 billion years ago, enough oxygen had been released to oxidize most of the methane in the earth's atmosphere. Methane was the most powerful greenhouse gas in the early atmosphere, and its destruction triggered the Huronian ice age, the first ice age of which we have any clear record. The combination of rising oxygen levels and plummeting temperatures was catastrophic for Archean life. Oxygen is an extremely reactive element, and it poisoned most of the organisms that had evolved in its absence. These retreated to environments sheltered from oxygen, where they remain today. New forms of life evolved that were resistant to the toxic effects of oxygen and could even put it to use to increase the efficiency of their cellular processes. Among these were the first eukaryotes.
Bacteria and archaea, collectively known as prokaryotes, have little internal structure (other than chlorophyll-bearing membranes in cyanobacteria.) Their DNA, the giant molecule that carries the blueprints of life, is a single circular pair of matching strands that floats more or less freely in the cell compartment. The circular form is an elegant solution to the problem of how to begin or end the DNA strand: The closed circle has no beginning or end! However, this naked strand of DNA is vulnerable to damage, and its transcription (the process of using the blueprints to manufacture proteins) is slightly unreliable. Transcription in prokaryotes is also subject only to simple controls. This means that prokaryotes mutate rapidly, which is not necessarily a disadvantage for single-celled organisms seeking to occupy as many ecological niches as possible. But it also means that prokaryotes cannot readily specialize, a prerequisite for multicellular organisms. While some prokaryotes form large colonies, these are not characterized by much specialization of roles.
The eukaryotes, by contrast, have cells with a complex internal
structure, including multiple separate compartments such as the
nucleus. Their DNA is organized into linear pairs of matching
strands called chromosomes. These begin and end with telosomes,
sections of DNA that bind particularly tightly between pairs to
prevent the DNA from unravelling. (Such unravelling seems to be
part of the natural aging process of multicellular organisms like
ourselves.) The organization of DNA into multiple chromosomes
within a special compartment makes for a much lower mutation rate
and more sophisticated control of transcription. This in turn
makes true multicellular life possible. Thus, modern eukaryotes
include all animals, plants, and fungi, as well as several groups
of complex single-celled organisms such as amoebas and ciliates.
In addition, virtually every lineage of eukaryotes carries the
basic genes for sexual reproduction, in which the organism carries
two complete sets of chromosomes for at least part of its life
cycle. One set of chromosomes can be swapped with another organism
of the same species, allowing a much greater degree of genetic
diversity within a species that is otherwise genetically stable.
This likely greatly hastened the process of evolution.
Just how eukaryotes emerged is still being worked out by
scientists. However, some of the internal compartments of
eukaryotic cells (including mitochondria, which power the cell,
and chloroplasts, which carry out photosynthesis) are about the
same size and shape as bacteria, and they even have their own
circular DNA. This strongly suggests that eukaryotes began through
endosymbiosis, where small prokaryotes took up residence
within a larger prokaryote. The relationship proved beneficial to
both, and the pairing evolved into a single organism. The
mitochondria and chloroplasts of modern eukaryotes are now
incapable of independent life, since many of the genes encoding
their essential proteins have moved to the nucleus of their host
cell. Whether the nucleus itself began with endosymbiosis, or
evolved by a different mechanism, is a subject of active research
and debate.
The oldest unambiguously eukaryotic fossils, of the red alga Bangiomorpha
pubescens, were found on Somerset
Island in the Canadian Arctic in rock beds about 1.0 billion
years old. However, acritarchs first appeared 1.6 billion
years ago. Acritarchs were single-celled organisms that were the
same size as modern eukaryotic cells, and there are hints of
membrane-bound nuclei in some acritarch fossils. However,
acritarchs became extinct around 500 million years ago and their
true nature is uncertain. There is evidence that eukaryotes may
have emerged even earlier: The earliest traces of organic
compounds characteristic of eukaryotic life are found in rocks
dating back to around the beginning of the Proterozoic, 2.5
billion years ago. Such traces of organic compounds are known as molecular
fossils. However, since almost all eukaryotes contain
mitochondria, and the function of mitochondria is to produce
chemical energy by oxidation, it seems likely that true eukaryotes
emerged in an environment where oxygen was already present. This
may well have been in proximity to cyanobacteria colonies that
produced a continual supply of the reactive element.
Paleoproterozoic Laurentia was a collage of crustal fragments,
most of which had formed during the Archean. There is much
that is still not known about the Archean Eon and about the
process of crust formation, but it is widely believed that the
continents started as small bits of continental crust. These
gradually assembled, sticking to each other when they were pushed
together by the oceanic conveyor. It is not known how long this
process took, and there are differences of opinion on whether
large continents existed yet during the Archean. However, some of
the continental crust that formed the heart of Laurentia included
possible miogeoclinal sedimentary beds, formed on the
passive margin of a continent following rifting. This suggests
there were earlier large continents that broke apart.
There is a fair amount of agreement among geologists that, from
the start of the Proterozoic on, the continents have assembled
into a supercontinent (containing 75% or more of the Earth's
continental crust) about every 750 million years or so. The
supercontinent then breaks up again. Perhaps this occurs because
of periodic changes in the pattern of convective flow in the
mantle that shifts the locations of the mid-ocean ridges. The
supercontinent itself may help trigger such changes, by trapping
heat in the underlying mantle. While geologists disagree over
whether a supercontinent existed during the Archean, there is good
evidence that a supercontinent assembled around 1.8 billion years
ago. This supercontinent has been given many names: Nuna,
Hudsonland, Columbia.
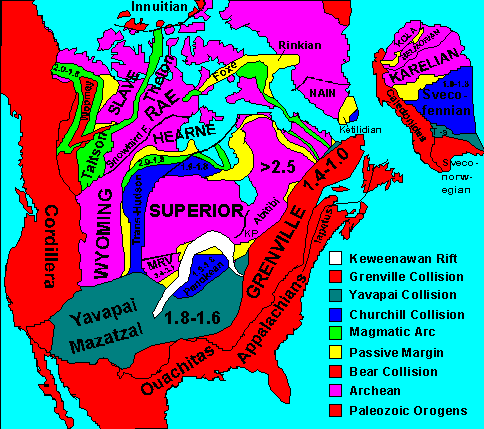
Archean rocks form the cores of the modern continents, which are known as the continental shields. Proterozoic rocks underlie much of the sedimentary rock in the continental platforms that surround the shields. Together the platforms and shields form the stable continental cratons.
No Archean rocks are found in New Mexico, because New Mexico didn't yet exist.
North America seems to have begun assembling out of smaller fragments of crust, which geologists call provinces, about 2.0 to 1.8 billion years ago. The largest of these fragments was the Superior Province, which took in the Great Lakes area and the adjoining parts of central and eastern Canada. Another was the Slave Province of northwest Canada, which had previously assembled out of three smaller provinces. At around 1.84 billion years ago, these provinces collided and merged to form Laurentia. Shortly afterwards, two more provinces merged with Laurentia to form the future Wyoming and Montana region. By 1.8 billion years ago, the western margin of Laurentia, which would face south today, ran roughly along what is now the Wyoming-Colorado border.
The difference between compass orientation today and in the past
is bound to complicate our story. Rather than constantly saying
things like "west, which would be south today", I'll tell the
story as if New Mexico was in its modern orientation, with only
occasional comments on what the actual orientation was at the
time.
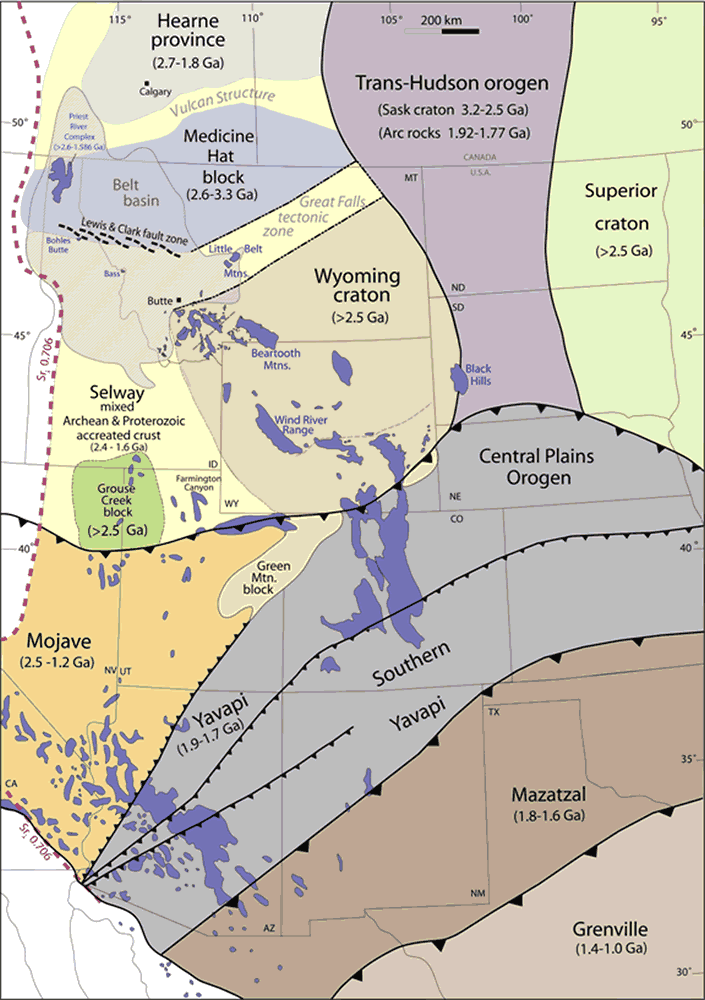
During the next four hundred million years or so — longer than
the time interval between the emergence of the first amphibians
and the present day — a mid-ocean ridge was active south of
Laurentia. The oceanic lithosphere spreading from this ridge
subducted under the southern margin of Laurentia, and a sequence
of microcontinents and oceanic island arcs carried by the
oceanic crust were brought up against the continent.
A microcontinent is a small patch of continental lithosphere. The largest modern examples are Madagascar and New Zealand, but microcontinents can be as small as individual islands like Socotra. It is possible that most of the continental crust of the Earth started out as microcontinents, which assembled to form large continents.
As we saw in the last chapter, oceanic island arcs are formed
when oceanic lithosphere subducts under oceanic lithosphere, as is
the case with many of the island chains of the western Pacific.
That this is most common in the western Pacific, where the ocean
basin is far from the mid-ocean ridge, suggests that this is a
phenomenon of cold oceanic crust. This crust more easily subducts.
The island arcs above these subduction zones consist of rock that
is less dense than oceanic crust, but more dense than typical
continental crust.
When microcontinents or island arcs are carried into a
destructive margin by the motion of the underlying oceanic
lithosphere, they are unable to subduct because of their low
density. If the microcontinent is quite small, the lighter crust
shears off the underlying upper mantle, sticking to the continent
on the other side of the subduction zone. Accumulated sheared
crust forms an accretionary wedge on the continental
margin. The confused mass of broken and deformed rock beds in the
wedge are spoken of as a melange. The Franciscan Complex
of coastal California is an example of a melange.
When a large microcontinent is drawn into a subduction zone, the collision is more violent, throwing up high mountains on both sides of the collision zone, which geologists call a suture. We see this process taking place in the Himalayas today. India was once a large microcontinent (or small continent, depending on where you choose to draw the line) that was carried into the southern coast of Asia and is now sutured to the Asian continent along the line of the Himalayas. The collision event itself is known as an orogeny, from the Greek ὄρος oros, "mountain" + γένεσις genesis for "creation, origin". The zone of deformed crust and mountain building along the suture is called an orogen.
The microcontinents and island arcs that merged with southern Laurentia between 1.7 and 1.6 billion years ago formed the Yavapai and Mazatzal Provinces, which reach from modern southwest Arizona to Michigan and includes most of Colorado and New Mexico. The large region of accreted material making up these provinces consists of a mosaic of blocks between about 10km and 100 km (6 to 60 miles) in size, separated by shear zones, which are narrow zones of high deformation. The oldest Precambrian rocks in northern New Mexico belong to Yavapai formations that are about 1.77 billion years old.
Because they represent two episodes of a long process of accretion along the southern boundary of Laurentia, the Yavapai and Mazatzal Provinces are sometimes grouped with with other accreted provinces from this time as part of the Transcontinental Proterozoic Provinces. Accretion was not limited to Laurentia; subduction took place all along the western coast of Columbia, and produced similar accretionary provinces on the margins of what are now the continental cores of the Baltic and the Amazon basin. This accretion process is one of the most significant continental crust forming events discernible in the geologic record. Some geologists have suggested that the best modern counterpart is southeast Asia, where the island arcs of Indonesia and Malaysia are being welded onto Asia as a result of subduction both on the south (Indian Ocean) and east (Pacific Ocean).
Map of the Jemez Lineament
showing young volcanic fields. Public
domain.
If you examine a geologic map of the southwest United States, you
will find a line of young volcanic fields stretched across New
Mexico and Arizona. These include the Raton
volcanic field, the Mora
volcanic field, the Taos plain, the Jemez
Mountains, Mount
Taylor, the Lucero
volcanic field, the Zuni-Bandera
volcanic field, the Springerville volcanic field, the White
Mountains volcanic field, and the San
Carlos volcanic field.
When plate tectonics was still quite new, geologists identified the Snake River Plain as a hot spot trace. Volcanoes repeatedly erupted over a fixed point in the mantle as the North American plate moved southwest over this mantle hot spot. This explanation made sense, since the youngest volcanoes are at Yellowstone (and are potentially still active) and the oldest, most thoroughly extinct volcanoes are far to the southwest, in northern Nevada. Geologist today still believe the Snake River is a hot spot trace, though there is debate among geologists about the exact nature of the hot spot.
The Jemez Lineament seemed to fit the same pattern. The volcanism
followed a path of similar length and direction, and some of the
volcanoes at the northeast end of the Lineament were obviously
very young. However, as the rocks along the lineament were
radiometrically dated, the hot spot theory for the Jemez Lineament
began to fall apart. There is no systematic progression in age
along the lineament. Volcanism began a little earlier towards the
center of the lineament, but quickly spread southwest and
northeast. This is not consistent with a hot spot.
It is now widely believed that the Jemez Lineament is an ancient
structure of some kind in the lower crust or upper mantle. The
most widely accepted explanation is that the Jemez Lineament marks
a hydrated subduction zone scar where, some 1.7 billion
years ago, active subduction took place along what was then the
continental margin. Accretion of additional island arcs then
jammed the subduction zone and active subduction shifted further
south. The relic subduction zone contains minerals with an
unusually low melting point, making it a fertile source rock for
production of magma.
The Jemez Mountains are located squarely on the intersection of
the Jemez Lineament with the western margin of the Rio Grande
Rift. The Rift is a region of the crust stretching from central
Colorado into northern Mexico, roughly along the valley of the Rio
Grande, where the crust began to be slowly pulled apart about 30
million years ago. We'll have much more to say about the Rio
Grande Rift later in the book. Deep faults mark the east and west
boundaries of the Rift, separating it from adjoining mountain
ranges, such as the Tusas,
the Sangre
de Cristo, the Sierra
Nacimiento, and the Sandia
Mountains.
The pattern of volcanism is not the only evidence for the
existence of the Jemez Lineament. Precambrian rocks north of the
lineament have maximum ages of around 1.77 billion years, as we've
seen. South of the Lineament, the maximum ages are around 1.7
billion years, and there are subtle differences in isotope ratios.
These are interpreted as different isotope model ages for
the two regions of crust. The steady decay of 147Sm to
143Nd in the earth's mantle, mentioned in the last
chapter, means that fresh magma extracted from the upper mantle is
increasingly enriched in 143Nd with the passage of
geologic time. The isotope model ages for rocks north and south of
the Jemez Lineament show that those south were formed from
material extracted from the mantle significantly later.
Another piece of evidence is the strength of magnetic fields measured by aeromagnetic surveys. Sedimentary rocks normally are very poor in magnetic iron minerals, making them "transparent" to magnetic fields. Thus an aircraft flying low over the ground can measure the magnetic field of the basement rock underlying the surface sedimentary beds, except in the immediate area of recent volcanism. Such surveys were originally carried out to find hidden ore bodies, but they also allow geologists to trace basement structures. Magnetic fields are anomalously high along the Lineament, suggesting a buried structure rich in magnetic iron minerals.
Finally, there is seismic profiling evidence for a deep structure coinciding with the Jemez Lineament. Seismic profiling was originally developed by the petroleum industry and is a way to get information about the subsurface rocks using sound waves. It is similar to probing the structure of the Earth using P-waves from earthquakes, but you don't have to wait for a nearby earthquake. Seismic profiling is carried out by setting out a network of seismic detectors and then generating sound waves using explosives lowered into a borehole, by dropping an extremely heavy weight into a borehole, or by placing a large, heavy metal plate on the ground and vibrating the plate at a carefully chosen frequency. Each approach has its advantages. The sound waves are reflected when they hit a boundary between rocks of different types, and careful measurement of the return times of the sound waves can be used to map out the rock layers below the surface. It's like sonar, but for use underground rather than in the ocean.
Seismic profiling of the Jemez Lineament reveals that deep rock beds on either side dip into the Lineament. This is more pronounced on the north side, and the general structure suggests that the Lineament is where the Yavapai plate to the north was overridden by the Mazatzal plate to the south. Some subduction took place, and the hydrated subduction zone scar may well be an excellent source rock for production of magma, because it likely contains abundant hydrous minerals.
The Jemez Lineament appears to have been severely deformed by motion along deep north-trending faults across north-central New Mexico. Most of this deformation likely took place during the Laramide Orogeny, a period of mountain building that peaked around 50 million years ago. Some reconstructions suggest that the Precambrian rocks of the Sierra Nacimiento were originally directly west of the Precambrian rocks of Sandia Crest, while the Precambrian core of the Sangre de Cristo lay well to the north.
... these waters have been recommended by Doctor Nagle, of Santa Fe, in many chronic diseases, and always with success.
— Lieutenant William G. Peck, 1847
North of Espanola,
beyond the confluence
of the Rio Chama and Rio Grande Rivers, lies Black
Mesa. The road to Chama skirts the west end of the mesa, and
a side
road, U.S. 285, turns along the north side of the mesa and
follows the Rio Ojo Caliente to the village
of the same name. Ojo Caliente, "Hot Pool" in Spanish, is the
location of hot springs near the river. The Spanish discovered the
springs early in their settlement of New Mexico, but the exposed
location, subject to Comanche raids, prevented permanent
settlement until 1868. In that year, Antonio Joseph, the first
Territorial representative to the U.S. Congress, built a bathhouse
at the springs. This has grown into a small resort favored by the
gentry of Santa Fe.
West of Ojo Caliente is a ridge of ancient rock, and to the northwest is Cerro Colorado, "Red Hill". These are the southernmost outliers of the Tusas Mountains, which separate the San Luis Valley and Taos area to the east from the Chama Valley to the west. Cerro Colorado is covered with pinon scrub forest, like much of the surrounding area, but as one proceeds north, the scrub gives way to ponderosa pine forest. Separate roads lead to La Madera and Tres Piedras, the gateways to the Tusas.
Ojo Caliente is located just beyond the northern boundary of the
Jemez region as depicted in our digital maps, and the northern
Tusas Mountains well beyond that. However, the rocks of the Tusas
Mountains, and of the Picuris
Mountains to their east, tell a story that is crucial to our
understanding of the Jemez Mountains, so we will venture north for
an extended visit.
The Tusas and Picuris Mountains are underlain by Precambrian
rocks of the Yavapai Province. The precise boundary between the
Yavapai and the adjoining Mazatzal Province has proven difficult
to pin down, but there is a zone 300 km (200 miles) wide that
seems to be transitional between the two provinces. The southern
edge of the transitional zone is roughly coincidental with the
southern edge of the Jemez Lineament. Its northern edge is
unusually sharp and well exposed in the Tusas Mountains and has
been thoroughly studied by geologists interested in the process of
continent assembly. This boundary is defined by a lithological
discontinuity where rocks assigned to the Yavapai Province
north of the discontinuity abruptly give way to rocks assigned to
the Yavapai-Mazatzal transition zone south of the boundary. The
lithological discontinuity is nearly coincident with a major
structural feature, the Spring Creek Shear Zone, which,
unsurprisingly, lies along Spring
Creek. This feature is probably younger than the rock beds
themselves, having likely formed around 1.4 billion years ago.
The Precambrian rocks of the Tusas and Picuris Mountains have been distorted and altered by geologic processes over the last 1.77 billion years, a process called metamorphosis.
Geologists divide rocks into three large families. Igneous
rocks form directly from magma. They include such rock types as
granite, which forms from silica-rich magma that hardens
underground; basalt, which forms from silica-poor lava that erupts
at the surface; and ash flow tuff, which forms from hot volcanic
ash. Sedimentary rocks form from beds of clay, sand,
pebbles, or other fragments of older rock, or of minerals
precipitated from large bodies of water, that are gradually
cemented together, usually by additional minerals precipitated
from ground water. They include rocks like sandstone, shale, and
limestone. Metamorphic rocks form from existing rock when
it is subject to heating that causes the rock to recrystallize
without actually melting. They include rocks like schist and
gneiss.
The heat and pressure required to form metamorphic rock is
usually found only deep underground. When metamorphic rocks are
found near the earth's surface, they are a strong indication that
tectonic forces have brought up rock that was once deeply buried,
a process geologists call exhumation or unroofing.
Exhumation is possible because of isostasy. Isostasy is the term for the balance between the weight of the mountains and the buoyancy of the thickened crust beneath them. As the mountains are worn down by erosion, the light crust beneath bobs upwards, raising new mountains to restore the balance. When you consider that continental crust underneath the Himalayas is around 100 km (60 miles) thick, versus 40 km (25 miles) for more normal continental crust, it is not hard to see that prolonged erosion of a high mountain range can bring rock to the surface that was originally very deep underground.
Metamorphic rocks are sometimes classified by the original igneous or sedimentary rock from which they formed (their protolith). Thus one speaks of metarhyolite, metabasalt, or metaconglomerate if it is possible to determine that the protolith was rhyolite, basalt, or conglomerate. However, as the degree of metamorphism increases, the original form of the rock becomes hard to discern. Such rocks are classified according to their mineral content and degree of foliation. A foliated metamorphic rock is one in which the minerals have segregated into distinct bands. Foliation shows the direction in which stresses were applied to the rock while it was undergoing metamorphosis, with the foliation typically lying perpendicular to the direction of greatest compression.
The mineral content of a metamorphic rock gives clues to the temperature and pressure at which the rock underwent metamorphosis. This is because different minerals are stable under different conditions. Geologists speak of characteristic combinations of minerals that point to particular temperature and pressure regimes as metamorphic facies. I won't go into these in any detail, because metamorphic rocks are uncommon in the Jemez. The Jemez is mostly composed of relatively young rocks that have not experienced deep burial.North of Spring Creek is Hopewell Ridge, which is composed mostly of rocks assigned to the Moppin Complex. These are among the oldest rocks found in northern New Mexico, with a radiometric age in excess of 1.75 billion years, and are typical of the Yavapai Province. Similar rocks are found around Gold Hill and north of Dalton in the Sangre de Cristo Mountains and are thought to be part of the same ancient block of crust, but displaced by motion along the Rio Grande Rift. The Moppin Complex consists of thick beds of mafic volcanic rock interbedded with occasional thinner beds of felsic volcanic rock and sediment. All have been metamorphosed.

Moppin Complex exposed at at Placer Creek.
36.695245N
106.2471211W
A particularly interesting feature of Hopewell Ridge is the
presence of magnetite schist. This was once prospected as iron
ore, but mining a limited quantity of ore so far from existing
rail lines is not economical.

Magnetite schist.
36
38.331N 106 8.072W
Magnetite schist is probably metamorphosed banded iron formation.
The presence of banded iron formation on Hopewell Ridge is one
indication that the Moppin Complex rocks were erupted in a marine
environment, as part of an island arc. Another indication is the
presence of well-preserved pillow basalt, erupted under
water, in Moppin Complex outcrops in the Iron
Mountain area.

Relict pillow structure?
36.723343N
106.2427357W
The isotope model age of the Moppin Complex is close to its
crystallization age, indicating that the rock is juvenile,
formed from magma freshly extracted from the mantle rather than
from reworked older crust. This distinguishes Yavapai rock from
the Archean core of Laurentia, where isotope model ages are often
much greater than crystallization ages, and is another indication
that the Yavapai Province formed by accretion of island arcs.
The Moppin Complex is bimodal, meaning that the volcanic rocks and sediments from which it formed included high silica and low silica magmas, but little intermediate magma. An exposure of feldspathic schist along Hopewell Ridge is an example of a felsic member of the Moppin Complex.


A feldspathic rock is composed mostly of fine grains of quartz
and feldspar. These are visible under the loupe, which also shows
smaller quantities of a mafic mineral, possibly mica or amphibole.
The rock here is schistose, having a laminated structure
as shown by the thin layers of the mafic mineral, and hence is
described as feldspathic schist.
The minerals in feldspathic schist are important characters in
our story, deserving of proper introductions.
Quartz is a mineral composed of silicon dioxide, SiO2.
We've seen quite a bit of quartz already, but we'll now examine
this important mineral more closely.
Silicon atoms prefer to covalently bond with four oxygen atoms. Each of these oxygen atoms shares a pair of electrons with the silicon atom, allowing the silicon atom to surround itself with a shell of eight electrons. This is a particularly stable structure for most light chemical elements. Each oxygen, in turn, prefers to covalently bond to two silicon atoms, which likewise allows the oxygen atom to surround itself with a shell of eight electrons. (Two pairs of electrons are shared with silicon atoms, and two the oxygen keeps to itself.) If we were living in a Flatland world of two dimensions, a quartz crystal might form as shown in the following diagram:
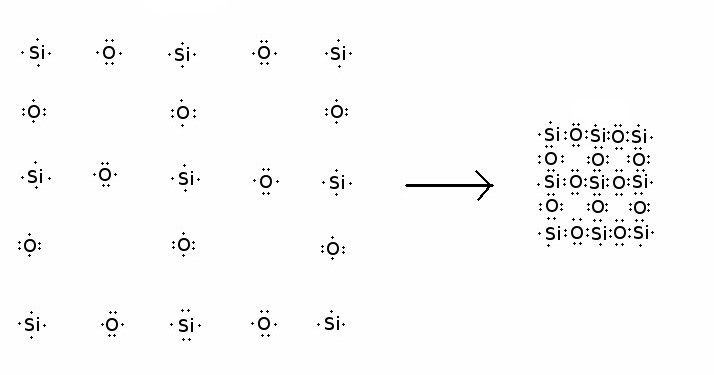
Electron-dot diagram of the formation of a hypothetical
2-D silica crystal
Each isolated silicon atom starts out with four outer shell electrons, and each isolated oxygen atom starts out with six outer shell electrons. When these atoms bond together to form quartz, the atoms in the interior of the quartz crystal all end up surrounded by the ideal shell of eight electrons.
Of course, we don't live in a two-dimensional world, and a real quartz crystal has a much more complicated three-dimensional structure. The four oxygen atoms bonded to each silicon atom lie at the corners of a tetrahedron, not in a flat plane. Nor do the two silicon atoms bonded to each oxygen atom form a straight line. Instead, because each pair of electrons in a filled electron shell wants to lie at a corner of a tetrahedron, the two pairs shared by silicon atoms lie at an angle close to 144 degrees rather than 180 degrees. (The angle is not the ideal 110 degrees of a tetrahedron, because the two silicon atoms repel each other enough to distort the tetrahedron.) This means that two silica tetrahedra sharing an oxygen atom lie at an angle of 144 degrees to each other. The tendency of the silica tetrahedra in quartz to find an arrangement in which the tetrahedra all lie at 144 degrees to each other is part of the reason for the peculiar structure of a quartz crystal, which is quite hard to visualize from two-dimensional images.
Nevertheless, I'll make an attempt here to explain the quartz structure, since quartz is so important. We'll start by examining the unit cell, which is the smallest piece of any crystal that contains the basis of its entire structure. A unit cell is always a parallelepiped; that is, it is a volume of space bounded by six faces with opposite faces parallel. For example, a cube is a parallelepiped in which the sides are squares meeting at right angles. In quartz, the unit cell has top and bottom that meet the sides at right angles, but the sides meet at angles of 60 and 120 degrees. The entire structure of a crystal can be generated from its unit cell simply by packing copies of the unit cell together so the faces all line up.
The unit cell of quartz is deceptively simple.
Each silicon atom is represented by a gray sphere and each oxygen atom by a red sphere, with the bonds shown as sticks joining the spheres. The spheres are not to scale, being shrunk down in size to show the bonds better; the spheres would be in contact in a scaled depiction, with the oxygen atoms much larger than the silicon atoms. There are thee silicon atoms and six oxygen atoms in the unit cell.
I know: You see six silicon atoms in the diagram. But the silicon atoms all lie on the faces of the cell, and so are shared with the neighboring cells. We could shift the boundaries of the unit cell so that our diagram shows just three silicon atoms -- the unit cell definition is not unique -- but this would not display the structure as well. The diagram shows bonds extending from the silicon atoms on the cell faces into the neighboring cells. If you examine the diagram for a few moments, you should be able to convince yourself that the pattern does indeed repeat itself, with (for example) the silicon atom on the top matching the silicon atom on the bottom. The silicon atom on each face has two bonds extending into the unit cell and two bonds extending into a neighboring cell. This makes the structure equally strong in all directions.
It can be startling to discover how this simple unit cell generates a wonderfully complicated crystal structure. To illustrate, we're going to show a single layer of unit cells, generated by lining up unit cells side to side and leaving the top and bottom free. Looking down on this layer, we see:
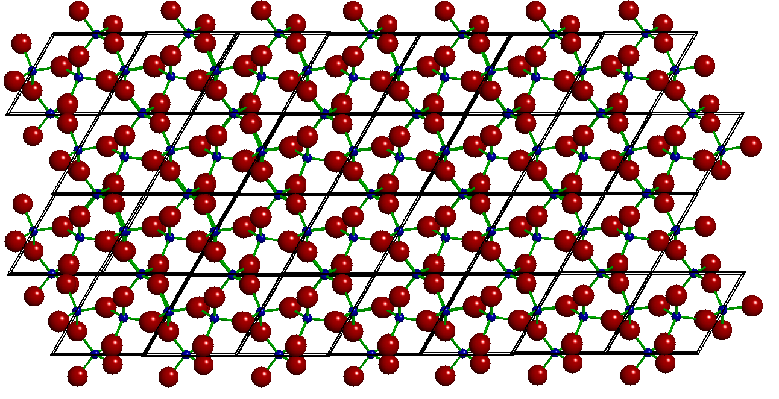
A single layer of alpha quartz
The unit cells are marked in this image. The full crystal consists of stacks of layers identical to this one. The silicon atom appearing as a small black sphere at the center of each unit cell is actually two silicon atoms, one on the top and one of the bottom face, that are vertically superimposed. These link the layers in the crystal.
The diagram shows that there are large channels running the length of the crystal; one such channel is marked in the version below.
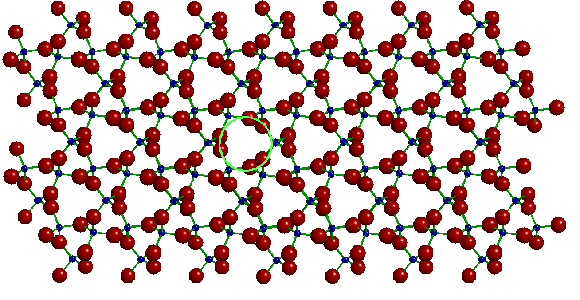
A single layer of alpha quartz with one of the channels
outlined
Because of these channels, a quartz crystal has a fairly open structure. This gives quartz a relatively low density, about 2.65 grams per cubic centimeter. (For comparison, the density of water is almost exactly 1.0 grams per cubic centimeter.) However, the strong three-dimensional bonding gives quartz the greatest hardness of any common mineral. Quartz is also chemically inert and very stable under the conditions found at the surface of the earth.
The Internet Quartz Page
has additional information on the wonderful and complicated
structure of quartz.
Feldspar is a mineral that is similar in structure to
quartz, but some of the silicon atoms have been replaced with
aluminum atoms. An aluminum atom has one less electron than a
silicon atom, and the missing electron must somehow be supplied if
an aluminum atom is to take the place of a silicon atom in the
crystal structure. Returning again to our Flatland world, the
formation of a feldspar crystal might take place as:
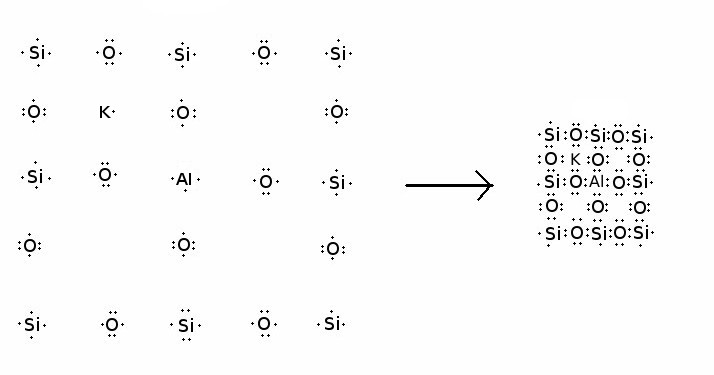
Electron-dot diagram of the formation of a hypothetical
2-D microcline crystal
A silicon atom has been replaced with aluminum, and a nearby potassium atom provides the missing electron needed to complete the structure. The potassium atom fits snugly into one of the openings in the structure, near the aluminum atom to which it donated its electron. As with quartz, the structure of a real feldspar in our three-dimensional world is much more complex and quite difficult to visualize from two-dimensional images. It is also not simply the quartz structure with added potassium; the silica and alumina tetrahedra still form a three-dimensional structure, but one that is subtly different from quartz, giving the potassium a little more room to fit in the structure. Quartz and feldspar, together with all other silicate minerals built on a basic three-dimensional network of interlocked silica and alumina tetrahedra, are called tektosilicates.
Atoms of sodium also readily donate an electron, while a calcium atom can provide two extra electrons to two aluminum tetrahedra. This gives us the three most common varieties of feldspar: potassium feldspar, KAlSi3O8; sodium feldspar, NaAlSi3O8; and calcium feldspar, CaAl2Si2O8.
I have described the bond between oxygen and silicon as covalent,
because the bond consists of a pair of electrons shared between
the two atoms. However, this is an idealization, like many things
in science. Oxygen is tremendously greedy for electrons: Only the
much less common element, fluorine, has a greater electron
affinity. So the sharing is unequal, with the electrons
being more tightly bound to the oxygen than the silicon. With
aluminum, the sharing is even more unequal. With other metallic
elements, the sharing is so unequal that the electrons effectively
have been lost to the metal and belong to the oxygen atom. Such a
bond is called an ionic bond, because both atoms have been
ionized: The metal atom, shorn of one or more of its
electrons, now has a net positive charge (making it a cation)
while the oxygen atom, having acquired two electrons from its
neighbors, has a net negative charge (an anion.)
The ions are bound to each other because of their overall opposite
charges. Geochemists find it convenient to speak of all
atoms in a crystal as if they have been ionized, even when the
bonding has considerable covalent character, as with oxygen and
silicon. I will follow this convention from here on.
Potassium feldspar comes in three separate varieties, or polymorphs, each of which is stable in a different range of temperature and pressure. The form stable at low temperature is called microcline.

MIcrocline feldspar from the Harding
Mine. Feldspar of this quality is rare in the Jemez. 36
11.557N 105 47.695W
Orthoclase is stable at elevated temperature, and sanidine
becomes the stable form at the highest temperatures. The high
temperature polymorphs are not uncommon in nature, because rapid
cooling after their formation can freeze the crystal structure
before it has time to convert to a lower temperature form. The
conversion from one polymorph to another can be thought of as a
kind of chemical reaction, and like many chemical reactions, it
takes place only at high temperature. Thus, volcanic rock often
contains sanidine, while intrusive rock contains orthoclase or
microcline.
Potassium feldspar is often found in the same rocks as quartz,
but it is easily distinguished by its tendency to fracture along
flat surfaces at nearly right angles, as in the photograph above.
This property is called cleavage. The number and relative
angles of cleavage planes are characteristic of any mineral.
Quartz has no cleavage planes, breaking instead along irregular
curved surfaces like those of thick broken glass. In addition,
quartz is usually nearly colorless and transparent while potassium
feldspar is translucent and often has a pink to brick red color.
Calcium and sodium freely substitute for each other in feldspar, forming what geologists call a solid solution series. This is because of the similarity in the sizes of sodium and calcium ions. The sodium ion has a radius of about 0.97 Angstroms (0.97 x 10-8 meters). The calcium has a very similar radius of 0.99 Angstroms. This is about 70% of the radius of an oxygen ion. Both ions fit very nicely into a site in the feldspar structure that is surrounded by eight oxygen ions. Because it has almost the same radius, a calcium ion easily substitutes for a sodium ion, so long as an aluminum ion simultaneously substitutes for a silicon ion to maintain charge balance. Calcium-sodium feldspar is called plagioclase, and plagioclase with all compositions from nearly pure sodium feldspar (albite) to nearly pure calcium feldspar (anorthite) is found in nature. Plagioclase can often be distinguished from potassium feldspar because its cleavage surfaces are striated, or marked by very fine parallel grooves.
Potassium does not easily substitute for calcium or sodium,
because its ions are significantly larger, at 1.33 Angstroms. It
can just fit into the feldspar structure, if the structure is
distorted to make more room for the potassium ions. In sanidine,
sodium substitutes fairly freely for potassium, but if the
feldspar cools slowly enough to convert to orthoclase, the sodium
tends to separate out into thin layers of albite to give what is
called perthitic feldspar. Most microcline is perthitic.
Ion size also explains why there is no such thing as magnesium or
iron feldspar. Both metals readily donate two electrons, like
calcium, and it seems like they might be able to replace calcium
in feldspar. However, the magnesium ion (with a radius of 0.66
Angstroms) and ferrous iron ion (with a radius of 0.64 Angstroms)
are significantly smaller than potassium, calcium, or sodium ions.
Ferrous iron and magnesium prefer to be surrounded by just six
oxygen ions, which is not possible in the feldspar structure.
However, small amounts of ferric iron (radius 0.63 Angstroms) can
substitute for aluminum (radius 0.53 Angstroms) in potassium
feldspar, with some distortion of the structure. This trace of
iron gives most potassium feldspar its characteristic pink to
brick red color.
The remaining components of our feldspathic schist outcrop are
mafic minerals, mica and amphibole. Mafic minerals are minerals
rich in iron and magnesium, and they tend to be dark in color.
A composition of quartz and feldspar with smaller amounts of mafic minerals is characteristic of granite, of which we'll see some beautiful examples later in this chapter. The feldspathic schist shown earlier has this granite-like composition, and these minerals are characteristically separated into layers in the rock. This thin layering suggests the presence of muscovite mica, which in turn is an indication of abundant aluminum in the rock. This suggests either an aluminum-rich granite protolith or a sedimentary protolith rich in clay, such as shale, of which we'll see many examples later. The thin layering is typical of shale and may indicate that this is actually a metashale.
You may have noticed that the Moppin beds, whether mafic or
felsic, are tilted almost vertically. This probably does not
reflect the original bedding. It is typical of rock that has been
subject to tremendous compressive forces associated with
continental suturing. The rock has nowhere to go but up, so it is
stretched almost vertically during metamorphosis.
Another clue to the history of northern New Mexico in the Precambrian is the presence of calc-alkaline igneous rocks within the Moppin Complex. The most widespread in the Tusas Mountains is the Maquinita Granodiorite, which has been dated at 1.755 billion years old. This is right in the age range typical of the Yavapai province, 1.7 to 1.8 billion years. This is likely the time of formation of the island arcs, somewhere offshore of Laurentia, that later accreted as the Yavapai Province.
The deposits along Hopewell Ridge are a gray fine-grained rock.

Maquinita Granodiorite on Hopewell
Ridge. 36.632287N
106.1246707W
Granodiorite is an intermediate-felsic intrusive igneous rock. An intermediate-felsic rock is an intrusive igneous rock with a fairly high silica content, between 63% and 69%, like dacite. It consists of crystals that are easily visible with magnification and are often obvious even to the naked eye. In a granodiorite, the crystals are found to be quartz and feldspar with some mafic minerals, much like granite. However, the feldspar in granodiorite is mostly plagioclase rather than alkali feldspar, which is the more abundant feldspar in granite.
In some locations the Maquinita Granodiorite has a much more granite-like appearance.

Maquinita Granodiorite near Iron
Mountain. 36.717427N
106.2471917W
The significance of the Maquinita Granodiorite is that, in addition to having a fairly high silica content, it is also moderately enriched in the alkali metals, potassium and sodium, and the alkaline earths, calcium and magnesium. Rocks that are enriched in this way, and which show other distinctive chemical characteristics (such as a high aluminum content and a tendency to steadily decrease in iron content as the silica content increases) are described as calc-alkaline.
Geologists speak of igneous suites, which are families of
igneous rocks having a similar origin. Each suite comes from its
own distinctive source rock subject to a particular degree and
type of partial melting. Calc-alkaline magma tends to form from
rocks that have already experienced some partial melting
(moderately depleted source rocks) in an environment that is more
oxidized and contains more water vapor than is the case with the
other common suite, the tholeiitic suite. The water vapor
alters the eutectic compositions, and the relatively high content
of oxygen means that, as the magma differentiates, much of its
iron is removed as magnetite crystallizes out. By contrast,
tholeiitic magma is poor in oxygen, and as it differentiates, the
iron content actually increases as magnesium-rich olivine
crystallizes out, instead of iron-rich magnetite.
The calc-alkaline family of rocks are characteristically erupted over subduction zones, where fluids "sweated" from the subducted slab provide water and oxygen, and the production of magma from the mantle wedge rapidly depletes the source rock. The presence of calk-alkaline rocks in northern New Mexico Precambrian formations further reinforces the idea that the Yavapai Province formed by accretion of island arcs along a destructive margin.
Both the calc-alkaline and the tholeiitic suites are described as subalkaline. Subalkaline rocks are notable for being silica saturated, meaning that there is enough silica in the rock for its entire alkali metal content to form feldspar. By contrast, alkaline rocks have a high enough content of alkali metals that they are silica undersaturated, so that some of the alkali metals are present as silicate minerals with a lower silica content than feldspar. Alkaline magmas are thought to be produced at a greater depth or from a lower degree of partial melting than tholeiitic magmas. We'll have more to say about silica saturation in a later chapter.
The Spring Creek Shear Zone neatly divides rocks of the Yavapai
Province to the north, which are assigned to the Moppin Complex,
from younger rocks of the Yavapai-Mazatzal transition zone to the
south, which are assigned to the Vadito and Hondo Groups.
Throughout this book, you'll find rocks identified by their
group, formation, or member. For example,
the Bandelier Tuff is one of the most important formations in the
Jemez area. It names a distinctive kind of volcanic rock found
throughout the Jemez that was formed by three similar caldera
eruptions 1.85, 1.61 and 1.25 million years ago. This formation is
divided into the La Cueva Member, the Otowi Member, and the
Tshirege Member, corresponding to the thee individual events. The
Bandelier Tuff is one of several formations making up the Tewa
Group, which includes most of the rock erupted in the Jemez in the
last two million years. Much of this book is organized around
describing formations in decreasing order of age.
One can subdivide members into beds and combine groups into supergroups. We will mostly refrain from doing so in this book. The important thing to remember is that a group consists of related formations, which in turn consist of related members. When a formation or member is composed almost entirely of a single rock type, it is described using that type, as with the Maquinita Granodiorite or the Bandelier Tuff.
A complex, such as the Moppin Complex, is a body of rock
that has been so distorted by metamorphism or igneous intrusion
that one can no longer assume that the rock beds are ordered by
age, with the younger beds at the top and the older at the bottom.
Many of the beds of the Moppin Complex have been so heavily folded
that the older beds now lie atop the younger beds.
With that digression on stratigraphy out of the way, let's return to our story.
About 1.71 billion years ago, the island arcs that had formed
south of Laurentia began accreting onto the continental margin.
This event, known to geologists as the Yavapai Orogeny, would
continue for another 30 million years. Accretion was interrupted
at least once by the formation of a back-arc basin.
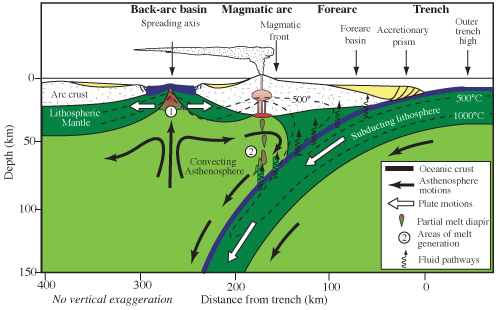
A back-arc basin forms in the crust above a subducting plate. It
may be caused by trench rollback, in which the trench
marking the point of subduction shifts in the direction of the
subducting plate. This stretches the overriding plate, sometimes
rifting the plate apart and forming what amounts to a very small
ocean basin behind the plate. Back-arc basins tend to close up
again, and this process may have taken place, possibly more than
once, during subduction under the Yavapai Province. Evidence for
the formation of a back-arc basin around this time is provided by
beds of pyrite-bearing chert near
Wheeler Peak in the Sangre de Cristo Mountains. The pyrite
and chert are thought to have formed in hydrothermal systems along
the axis of the basin. Geologists have named this basin the Pilar
basin, and the first set of formations laid down in the Pilar
basin have been assigned by geologists to the Vadito Group.
As the crust thinned and rifted under the Pilar basin, basalt
lava erupted across the basin floor. This would subsequently be
metamorphosed to amphibolite, which is rock rich in amphiboles
and plagioclase feldspar. Amphibolite of the Vadito Group is
exposed in the southern Tusas Mountains, where its dark color
stands out in the lower slopes of Mesa de la Jarita ("Jar Mesa")
east of Ancones.

Vadito amphibolite exposed in the slopes of Mesa de la Jarita.
36.4266743N
106.0667167W

Vadito amphibolite exposed in the slopes of Mesa de la Jarita. 36.435038N
106.063763W
Vadito amphibolite is also exposed to the east, in the Picuris
Mountains, a part of the Sangre de Cristo Range. Amphibolite
beds are exposed above the Harding Mine, where the dark
amphibolite contrasts sharply with the light pegmatite.

Vadito amphibolite exposed above Harding Mine. 36.1940773N
105.7997917W

Vadito amphibolite. 36.1940773N
105.7997917W
Chemical analysis of Vadito amphibolite shows a composition very
close to hydrothermallly altered basalt. However, the rock has
been recrystallized from a very fine-grained extrusive rock to a
coarse-grained metamorphic rock with visible crystals of
plagioclase and amphibolite.
Amphiboles are members of the family of silicate minerals called inosilicates, which are characterized by long chains of silica tetahedra. Amphiboles are those inosilicates in which the each silica tetrahedron is joined to two or three neighbors so that the silica backbone consists of two parallel chains of tetrahedra joined together:
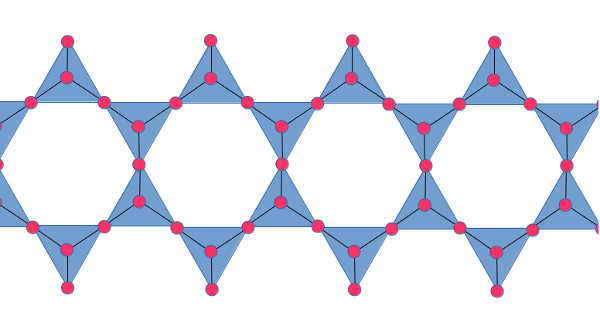
As with other silicate minerals, it is possible for aluminum to
substitute for some of the silicon. Even with no aluminum
substituted in the chain, additional metal ions are required to
balance the negative charge of the backbone. As with mica, these
are accompanied by hydroxyl groups. Pairs of double chains face
each other, with the apical oxygens on the inside bonded to a
strip of metal ions. Each such combination of two double chains
bonded by metal ions looks a little like an I-beam in
cross-section. The "I-beams" then interlock, with additional metal
ions holding the "I-beams" in place.
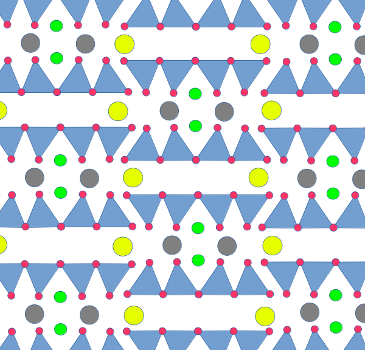
The metal ions holding the pairs of double chains together are
shown in gray, with the associated hydroxyls in green. The metal
ions locking the resulting "I-beams" together are shown in yellow.
The amphiboles show two cleavage planes corresponding to lines
drawn through the empty spaces above and below each "I-beam".
Hornblende is a rather general term for amphiboles rich in iron. A typical formula would be Ca2Fe5Si8O22(OH)2. The grey ions in the previous diagram would then be iron and the yellow ions would be calcium. However, magnesium substitutes freely for iron, sodium substitutes for calcium, and aluminum can substitute for both iron and silicon, producing a wide range in compositions.
As it continued to deepen, the Pilar basin became a trap for
sediments. The first sediments to accumulate were deposited in
deep water, were interbedded with some felsic volcanic rocks, and
contained a fair amount of silt and clay. These beds take the form
of micaceous schist, conglomerate, dirty quartzite, and
metarhyolite. All are close to 1.7 billion years in age. They are
described as supracrustal rocks of the Yavapai-Mazatzal
transition zone. Supracrustal rocks are rocks deposited on an
existing basement.
Some of the more silicic beds were metamorphosed to muscovite schist.

Vadito muscovite schist. 36.195188N
105.7853422W
The small crystals are a mineral called staurolite. We'll have
more to say about it presently. The three very large crystals,
each several centimeters across, are a mineral called andalusite.
Andalusite is aluminum silicate, Al2SiO5.
Just as potassium feldspar has three polymorphs, so aluminum
silicate has three polymorphs; andalusite is the form stable at
low pressure and moderate temperature.
The gray color and thinly layered character of the surrounding matrix is due to a high content of muscovite. Muscovite is a common mineral in both igneous and metamorphic rocks.
The next sample is also Vadito schist, but here the conditions of
metamorphism changed such that some of the andalusite was replaced
by staurolite.

Andalusite replaced by staurolite. 36.203555N
105.8151637W
Most of the black andalusite crystals have been replaced with
brown staurolite. This involved an increase in temperature and
pressure, likely due to increasing depth of burial, accompanied by
migration of water into the andalusite. Thus a crystal with
composition Al2SiO5 was replaced with a
crystal with composition Fe2Al9O6(SiO4)4(O,OH)2,
meaning that aluminum was leached out of the crystal as water was
added. Iron was probably already present in the mineral as an
impurity. In other places, the andalusite was replaced with
cordierite, which is likewise poorer in aluminum.
Muscovite belong to a family of silicate minerals called phyllosilicates. In a phyllosilicate, the silica tetrahedra are joined at only three of their corners, forming sheets of tetrahedra. Unlike the structure of quartz or feldspar, which is tough to depict in a two-dimensional image, it is easy to depict the structure of a phyllosilicate:
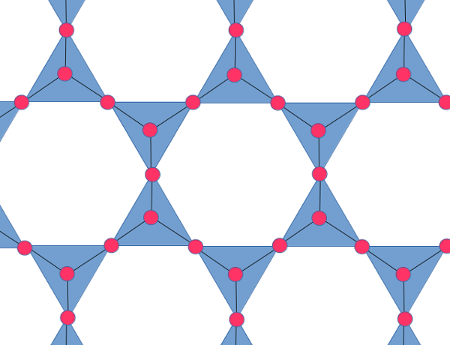
This graphic is drawn from the perspective of someone looking
directly down on a sheet of silica tetrahedra. Three of the oxygen
ions in each tetrahedra are shared; the fourth sits by itself at
the tip of each tetrahedron, as shown here. This fourth oxygen ion
is described as an apical oxygen ion. The overall structure is of
layers of interlinked rings of silica tetrahedra.
From a chemical standpoint, this structure is incomplete. The
apical oxygen ions are only connected to one silicon ion. In
addition, in muscovite, one silica tetrahedron in four is replaced
by an aluminum tetrahedron, which makes the structure even more
negatively charged. As with feldspar, the negative charge is
balanced by metal cations.
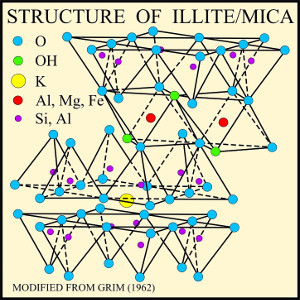
Muscovite structure. U.S.
Geological Survey
Muscovite is composed of layers of triple sheets. The upper and lower sheet of each layer is a phyllosilicate sheet. The sheets are oriented so that they face each other, with the apical oxygen ions on the inside. Between the phyllosilicate sheets is a sheet of aluminum hydroxide, Al(OH)3, a substance which in pure form crystallizes as the mineral gibbsite. A gibbsite sheet is not unlike a phyllosilicate sheet, but with rings of aluminum ions joined by hydroxyl (OH-) ions such that each aluminum ion is bonded to six hydroxyl and each hydroxyl to two aluminum ions. The apical oxygen ions of the outer phyllosilicate sheets bond to the gibbsite sheet by replacing some of the hydroxide ions. It's almost like a sandwich, with the two phyllosilicate layers as the bread and the gibbsite as the sticky layer of peanut butter or marmite that holds the two slices of bread together.
The gibbsite sheet starts out electrically neutral, while the two phyllosilicate sheets start out negatively charged because of their apical oxygens. Each hydroxyl ion that is replaced by an apical oxygen ion reduces the negative charge, and for two pure silica phyllosilicate sheets fully bonding to one gibbsite sheet, it all balances out to make the triple layer neutral. Stacks of such triple layers make up the uncommon mineral, pyrophyllite.
In muscovite, one in four silicon ions in the phyllosilicate
sheets are replaced by aluminum, giving the triple layer a net
negative charge. Each ring in the phyllosilicate layer forms a
kind of cup in the outer surfaces of the triple layer, which is
lined with oxygen and hydroxyl ions. This negatively charged cup
is an inviting location for a potassium ion to sit. The
neighboring triple layers have corresponding cups that fit to the
potassium ions and bind the sheets together, producing a structure
with no net charge. The binding of triple layers by potassium
makes muscovite significantly harder than pyrophillite.
The family of phyllosilicate minerals which share the three-layer
structure of muscovite are known as micas. Different mica
minerals substitute different metals for aluminum and potassium,
but have the same basic structure.
The binding by potassium ions is not particularly strong. As a
result, mica is easily split between triple layers. This gives
mica crystals a single perfect cleavage plane. It is possible to
split mica into very thin sheets, which have been used for
insulation, as a dielectric in electronic components, and even as
a substitute for glass.

Muscovite is an aluminum-rich mineral, with equal numbers of aluminum and silicon ions in its structure. This contrasts with alkali feldspar, which has three silicon ions for every aluminum ion. The presence of muscovite in granite is a indication that the granite is peraluminous, rich in aluminum. Muscovite in a metamorphic bed suggests the protolith was enriched in clay, which has a high aluminum content.
Following the deposition of sand and shale in the Picuris area, geologists discern subtle signs of a pause in deposition. However, deposition continued in the Tusas area, where the aluminum-rich shale beds are overlain by another striking conglomerate, the Big Rock Formation.

Big Rock Conglomerate. 36
32.944N 106 05.654W
The Big Rock Formation is highly foliated, showing it was strongly compressed and deformed. However, there are numerous large quartz pebbles in the conglomerate which are almost undeformed. This shows the rest of the rock was much softer than the quartz. This likely was gravel in river channels in a muddy floodplain, probably close to volcanoes erupting silica-rich ash.
As the crust on the southern coast of Laurentia buckled and
thickened, magma rising to the surface became increasingly rich in
silica. Silica-rich volcanic ash began to be deposited in the
Pilar basin.
In the Tusas area, the ash became the Burned Mountain Formation. This is a metarhyolite with a radiometric age of 1.70 billion years, significantly younger than the Moppin Complex. It was originally mapped as dikes and vents intruding the Moppin Complex, such as those along Placer Creek south of Hopewell Lake.

Burned Mountain Formation. 36.6933581N
106.2493222W Public
domain
Here is a particularly fine exposure in a dike intruding Moppin Complex beds at Iron Mountain.

Burned Mountain Formation dike at Iron Mountain. 36.724738N
106.2445007W
The formation has since been redefined to include most of the younger beds of the Vadito Group in the Tusas Mountains. These include outcrops on Mesa la Jara.

Burned Mountain Formation on Mesa la Jara. 36.724738N
106.2445007W
There are particularly fine outcroppings in the vicinity of Ojo
Caliente, whose radiometric age leaves little doubt they belong to
the Burned Mountain Formation.

The ridge from which this panorama was photographed is northwest
of the resort, and the panorama begins looking to the northeast.
The ridge itself, which continues to the south, is nearly the
southernmost Precambrian exposure of the Tusas Mountains. The peak
to the right in the panorama is Cerro Colorado, the southernmost
peak of the Tusas, underlain also by Burned Mountain Formation.
Here's a closer look at this rock.

Xenolith. From a point just southwest of the hill top of
the previous panorama.
This boulder contains a patch of darker material, which is likely a xenolith. A xenolith is a bit of country rock picked up by a body of liquid magma, which does not quite melt and remains distinct from the magma. In this case, the darker color and coarser grains of the xenolith suggest it is a mafic rock, possibly from the lower crust or upper mantle.
Exposures of Burned Mountain Formation are extensive in the southern Tusas Mountains. They form beds that lie parallel to underlying metasedimentary beds, suggesting that they formed from metamorphosis of high-silica volcanic ash flows. The ash came from nearby continental arc volcanism, probably through dikes and vents like those that intrude the Moppin Complex.
Among the beds of the Burned Mountain Formation are aluminum-rich
schists, which were once mined for kyanite on Mesa
de la Jarita.

Kyanite mine on Mesa de la Jarita. 36
32.657N,106 04.920W

Kyanite. 36
32.657N,106 04.920W
Kyanite is aluminum silicate, Al2SiO5. The
best samples have a striking pale blue color, of which there is
just a hint in these samples. Kyanite is the polymorph stable at
lower temperatures and high pressures than andalusite, which is an
indication of the metamorphic conditions where this rock
recrystallized.
The protolith for the kyanite must have been highly enriched in aluminum. Geologists who have closely examined these beds have found evidence that the enrichment occurred in a hydrothermal system associated with Vadito volcanism.
In the Picuris Mountains, the younger beds of the Vadito Group
correlating with the Burned Mountain Formation are assigned to the
Glenwoody Formation. This is most composed of quartz schist, and
is prominently exposed in the Pilar Cliffs.

Glenwoody Formation.
36.2658623N
105.7956017W
The formation varies from moderately dirty quartzite to muscovite schist.

Glenwoody Formation muscovite schist. 36.2658623N
105.7956017W
The Glenwoody Formation is thought to be heavily metamorphosed
rhyolite erupted as the back arc basin began to close up again.
There is a distinctive pink manganese zone near the upper part of
the formation that we'll say more about presently.
The village of Tres Piedras ("Three Rocks")
is the eastern gateway to the Tusas Mountains. To the east is the
sagebrush plain of the Taos Plateau, while the village itself is
forested with ponderosa pine. The town derives its name from three
large outcrops of "granite", which record the end of the Vadito
back-arc basin.
The high-silica ash of the Burned Mountain and Glenwoody Formations had its source in chambers of felsic magma deep in the crust, the largest of which formed the Tres Piedras Orthogneiss. These intrusions cut across both the Moppin Complex and the Vadito Group. Such intrusions along suture zones, which help join continental plates together, are sometimes described as stitching plutons. (Pluton is a general term for a large body of intrusive rock, derived from Pluto, the Greek god of the underworld.)

Tres Piedras Orthogneiss.
36.659268N
105.9841607W

Tres Piedras Orthogneiss up close. 36.6520517N
105.9686705W
The orthogneiss is a mixture of quartz and feldspar with thin
bands of mafic minerals. Laboratory analysis shows that the Tres
Piedras Orthogneiss has the composition of a true granite, and a
radiometric age of 1.693 billion years.
Part of the contact between the Tres Piedras Granite and the Moppin Complex is exposed in a road cut just west of the village.
Contact between Moppin Complex (left) and
Tres Piedras Granite (right). 36
39.155N 10558.697W
|
Under the loupe, the schist appears to be mostly black amphibole
with a scattering of white feldspar and an occasional dark garnet.
This contrasts with the mixture of quartz and feldspar with the
occasional flake of mica that makes up the orthogneiss.
Another, smaller, intrusion is exposed at Tusas Mountain. The age
of this rock is controversial, but the best recent measurement
gives the age as about 1.693 billion years, and it is likely part
of the Tres Piedras Orthogneiss.
The other major group of formations south of the Spring Creek
Shear Zone are the Hondo Group. These are all less than 1.7
billion years old. Most of the exposures consist of a very
clean quartzite called the Ortega Quartzite, which was laid down
towards the end of the Yavapai Orogeny on a gently sloping surface
eroded out of the older Moppin Complex and Vadito Group beds.
The Hondo Group is interpreted as continuing deposition in the
back-arc basin, but transitioning from deep water deposition to
shallow marine and fluvial deposition.
The Ortega Formation originally consisted of cemented grains of almost pure quartz, deposed around 1.680 billion years ago. It has since undergone metamorphosis to a very tough rock called quartzite. Quartzite is composed of almost pure quartz crystals packed densely together, which differs from sandstone, which consists of individual rounded grains of quartz with considerable pore space (which is sometimes filled with other minerals). Quartzite forms because quartz under stress is slightly more soluble than quartz that is not under stress. When sandstone comes under great pressure, the points where the individual grains touch take up the stress, which causes the quartz to dissolve away at the contacts. It is redeposited where there is less stress -- in the pore spaces between the original grains.
Extensive outcroppings of Ortega Quartzite are found in the Tusas
and Sangre de Cristo Mountains.
Kiowa
Mountain in the Tusas Range is underlain by Ortega
Quartzite.

Ortega Quartzite crops out throughout the Ortega Mountains of the southwestern Tusas, which is its type locality. A type locality is where a particular rock formation is fully exposed and can be described to other geologists. Formations are named for a location at or near the type locality. Here is one outcropping of particularly clean muscovitic quartzite.

Muscovite quartzite. 36
25.682N 106 0.735W
Under the loupe, this stuff does indeed look like almost pure quartz, with just a few flakes of light mica. This is what is meant by "clean" quartzite.
Ortega Quartzite is also found in the Picuris Mountains to the
east, where it is somewhat different in color but equally clean.
Here it underlies most of the ridges of the mountains.

Ortega Quartzite. 36
12.210N 105 54.545W
The contact between the Vadito Group and the Hondo Group is spectacularly exposed in the Pilar Cliffs in the gorge of the Rio Grande.

PIlar Cliffs. 36
15.738N 105 48.262W
The near-vertical cliffs are exposures of quartzose schist of the
Glenwoody Formation, Vadito Group. The cliffs are capped with
Ortega Quartzite. The contact between the two, which is difficult
to make out in this photograph but is very distinct at close
range, shows features consistent with a ductile shear zone. The
beds of the Glenwoody Formation just below the contact have a pink
coloration and are anomalously rich in manganese. This magnesium
zone can be traced throughout the region and marks the upper
boundary of the Vadito Group. Geologists are unsure what produce
the zone; two possibilities are that it was produced by widespread
hydrothermal activity or that it is a regional erosional surface.
The ductile shear zone shows that the Ortega Quartzite was
displaced south relative to the Glenwoody Formation. One
possibility is that this is related to mountain collapse,
where compression from subduction was reduced and the associated
arc range sagged back down. The other is that this is related to
the Picuris Orogeny at 1.4-1.5 billion years, which we'll come to
presently.
The very clean character of the Ortega Quartzite has been a
puzzle for geologists. The formation of sandstone of nearly pure
quartz in the Phanerozoic Eon almost always required repeated
cycles of erosion and deposition, but the Ortega Quartzite seems
to have been a first-cycle quartz unit, formed from
sediments eroded directly from igneous rock. Some geologists have
suggested that the unique chemistry of the ocean and atmosphere
1.6 billion years ago, when oxygen was just beginning to replace
carbon dioxide and hydrogen sulfide, may account for the rapid
chemical weathering of the sediments. Others point to the lack of
any vegetation other than microbial mats or to extreme
environmental conditions of wind and tides. The early Earth
rotated more rapidly than today, the Moon was closer, and both
make for more extreme tidal conditions.
Although the Ortega Quartzite is almost pure quartz, it does contain some magnetite, and there are also occasional thin beds of what must once have been clay. These were so severely metamorphosed that they recrystallized as the high temperature polymorph of aluminum silicate, sillimanite.

Ortega Quartzite with sillimanite vein.
36
15.776N 105 47.610W

Ortega Quartzite with sillimanite vein.
36
15.776N 105 47.610W
Compared with the kyanite shown earlier, the sillimanite is coarser and more translucent. It also lacks the bluish coloration.
Geological history shows a repeated pattern of fluctuating sea
levels. Geologists believe that during times of rapid mid-ocean
rifting, the hot, buoyant oceanic crust produced at highly active
mid-ocean ridges displaces enough ocean water to flood the
continents. The advance of the shoreline into the continental
interior is called a transgression; the subsequent retreat
of the shoreline off the continent is called a regression. The
Ortega Formation is a transgression sequence deposited as
beach sand as the sea advanced into the Pilar basin.
Although the Ortega Quartzite dominates the Hondo Group in the Tusas Mountains, other Hondo Group beds are present in the Picuris Mountains. The Rinconada Formation is probably slightly younger than the Ortega Quartzite. It includes some impressive muscovite schists.

Rinconada Formation. 36
19.646N 106 03.324W
The Rinconada Formation is famed for its staurolite beds.

Rinconada Formation. 36
15.738N 105 48.262W

Rinconada Formation. 36
15.738N 105 48.262W
Staurolite is an index mineral, characteristic of a particular metamorphic environment. The geologist George Barrow first identified distinct zones of increasingly highly metamorphosed mudstone in the Scottish Highlands, now called the Barrovian zones. Each of these zones is marked by the appearance of a new index mineral. The earliest stage of metamorphosis produces chlorite, a mineral similar to mica. Further metamorphosis at increasing pressure and temperature produces biotite mica, then garnet, then staurolite. Later comes kyanite and sillimanite, the last occurring as the rock approaches the melting point at great depth.

Rinconada Formation. 36
15.738N 105 48.262W
Individual staurolite crystals easily weather out of the less
resistant matrix, and these are often "twinned" crystals like the
ones shown above. Most twins are pairs of rodlike crystals that
interpenetrate at an angle of about 60 degrees. Rare, and more
valuable, are twins at right angles, which form so-called "fairy
crosses." There is a single imperfect fairy cross in these
samples, third from the left in the top row.
Staurolite is a fairly common mineral, but crystals this well
formed are unusual. The staurolite zone represents a narrow range
of temperature and pressure, near 580 Centigrade at a depth of
about 28 km (17 miles). Outside this zone, staurolite is
unstable. A difference of 15 degrees Centigrade in either
direction prevents staurolite from crystallizing. In addition,
only very aluminum-rich sediments produce staurolite, even when
they are in the right temperature and pressure zone. Anything less
aluminum-rich produces only garnet. So the Pilar staurolite beds
are pretty unusual.
You may be wondering why, if staurolite is unstable outside a
narrow temperature range, we see any in surface rocks. The answer
is that both the formation and the destruction of mineral crystals
in metamorphic rock is slow even at high temperature, and very
slow indeed at low temperature. If the rock is brought rapidly to
the surface by tectonic forces, so that the rock is rapidly
cooled, unstable minerals can survive for a very long time.
The counterpart of the Tres Piedras Orthogneiss in the Picuris Mouintains is the Puntiagudo granite porphry.

Cerro Puntiagudo. 36.1951223N
105.8551907W

Cerro Puntiagudo granite porphyry
This rock has a radiometric age of 1.684 billion years, just a
little younger than the Tres Piedras Orthogneiss. However, it is
more strongly sheared, though only to the point where there are
hints of banding. It crops out throughout the southernmost Picuris
Mountains, forming a high ridge that is cut by the gorge of Embudo
Creek.
Having ventured north for clues to wider events during the Paleoproterozoic, we now examine the Paleoproterozoic rocks of the Jemez region itself.
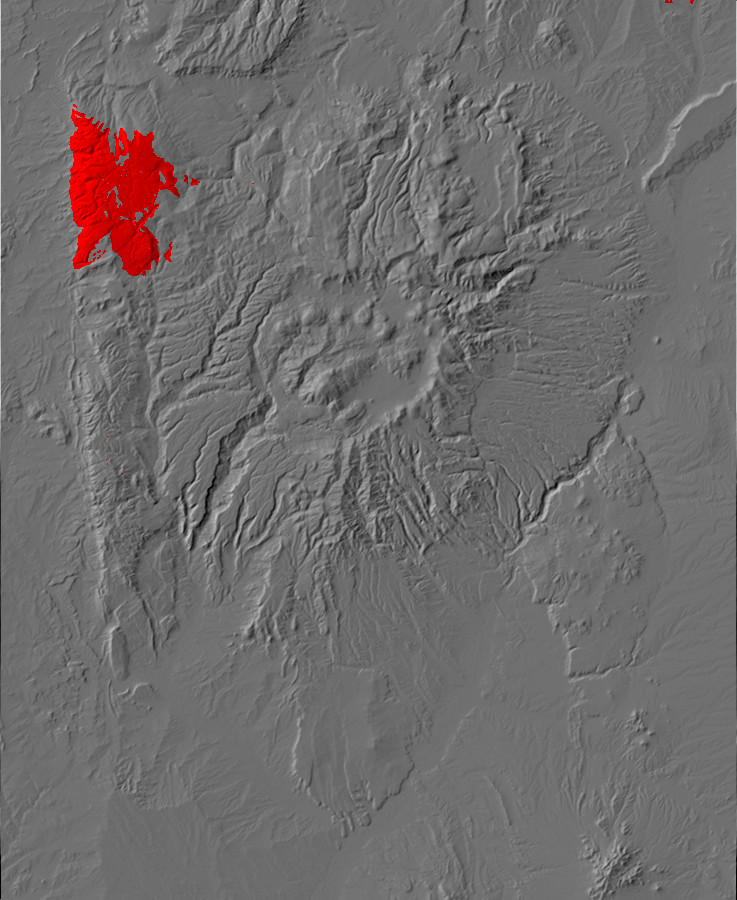
Relief map of the Jemez with Yavapai outcroppings
highlighted in red.
At the westernmost edge of the Jemez is a range of very old
mountains that runs almost directly north and south. This is the Sierra
Nacimiento. To its west is the San Juan Basin of the
Colorado Plateau, and moisture picked up by the prevailing winds
as they cross this long stretch of flat ground is wrung out over
the Sierra Nacimiento. The rainfall supports lush forests of
ponderosa pine and other conifers that mantle peaks and valleys
softened by erosion. Between the Sierra Nacimiento and the Jemez
Plateau to its east is the valley of the Rio Guadelupe and Rio de
las Vacas. This is a favorite camping area for weekend visitors,
but also supports small herds of cattle and some logging.
One may even encounter Hispanic cowboys driving their cattle along
the few highways in the area, as they have done for over three
centuries.
Señorita Canyon cuts across the Sierra Nacimiento east of Cuba,
separating the northern third of the range from the remainder of
the mountains. The northern Sierra Nacimiento, also known as the
San Pedro Mountains, is an area of parks (mountain valleys) and
gentle hills forming a high plateau that reaches to a maximum
altitude of 3232m (10,605').
Though part of the Jemez Mountains, the Sierra Nacimiento has a
very different origin and history from the rest of the region. The
bulk of the Sierra Nacimiento Mountains is composed of Precambrian
rocks that have been repeatedly thrown up by tectonic forces
during the Phanerozoic Eon. In the northern Sierra Nacimiento,
these include the oldest rocks in the Jemez region.
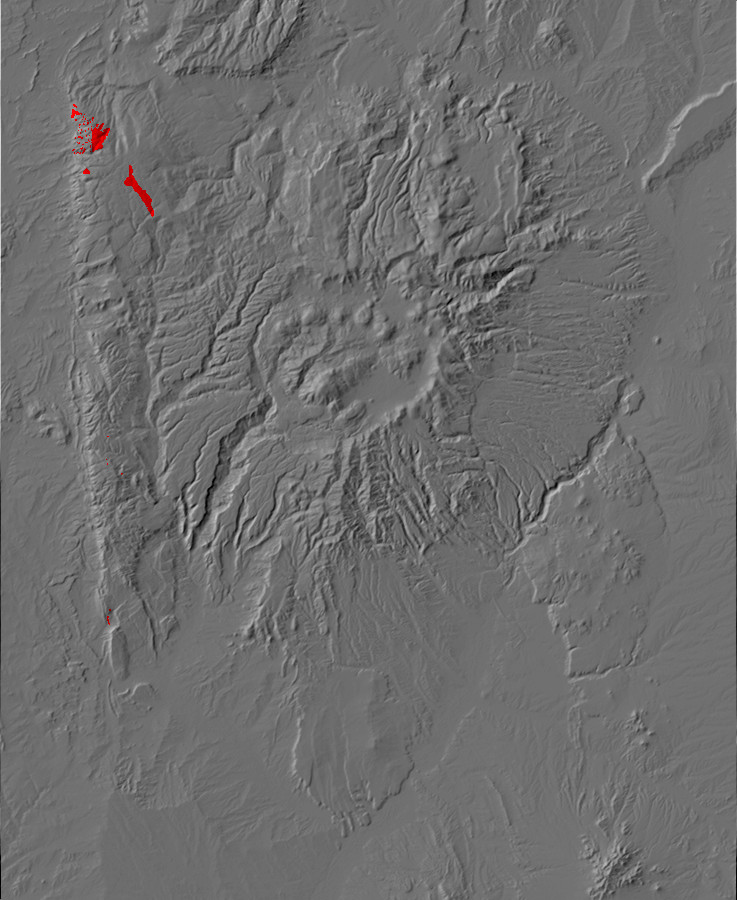
Relief map of the Jemez with San Pedro metamorphic
outcroppings highlighted in red.
The San Pedro Mountains are a patchwork of plutons, most of which
have never been radiometrically dated. There are only isolated
remnants of the older metamorphosed sedimentary and volcanic beds
that the plutons intruded, and the relative ages of the plutons
must be inferred from the contact relationships between them. Such
radiometric ages as we have suggest that the older metamorphic
rocks are correlated with the Vadito Group.
These rocks are exposed in Rito de las Perchas, a pleasant mountain park (grassy valley) in the San Pedro Parks Wilderness accessible by trail. The bulk of the metamorphic beds here are a metarhyolite not unlike the Burned Mountain Formation or the Glenwoody Formation. The area is gentle rolling hills and exposures tend to be poor.
Relief map of the Jemez with San Pedro tonalite
outcroppings highlighted in red.
One of the older plutons is composed of a rock variously described as tonalite, granodiorite, or quartz diorite. All imply a rock with moderate to high quartz content and much more plagioclase feldspar than alkali feldspar. According to the geologic map, this is exposed along the Rito de las Perchas and in the road cut on the forest road to the south.
San Pedro tonalite along Rito de las Perchas. 36.0554323N 106.808372WThe sample is unusually rich in dark iron minerals, mostly
biotite. These have produced some staining, but the feldspar is
white plagioclase rather than pink potassium feldspar. There is
also abundant muscovite. The rock is not actually deficient in
potassium, but is rich in aluminum, which has tied up the
potassium as micas rather than potassium feldspar.This hints at an
origin from melted clay-rich sedimentary rock. Such granites are
classified as S-type granites by geologists.
Relief map of the Jemez with quartz monzonite
outcroppings highlighted in red.
The San Pedro Quartz Monzonite is an intrusive rock containing
roughly equal quantities of alkali and plagioclase feldspar and
between five and twenty percent quartz. Like the Maquinita
Granodiorite, the San Pedro quartz monzonite is considered a
calk-alkali rock and, with an age of 1.73 billion years, it is
similar in age to the Maquinita Granodiorite.
This outcrop has a small shear crossing it.
This is a zone in which the rock has been deformed, forcing the
crystals to align into bands. The rock to the south (right) shows
no signs of deformation. That to the north (left) gradually
transitions from the sheared appearance in the center of the
photograph to an undisturbed texture. Such shears are associated
with deep crustal movement, with the rock on opposite sides moving
past each other. This resembles a fault, but occurs at such great
depth that the rock flows rather than fractures. In other words,
this occurs at depths below the brittle-ductile transition.
Similar shears trending east to east-northeast are common enough
in this area to define a shear zone, called the Nacimiento Creek
Shear Zone.
Nearby is a big patch of more mafic rock.
This is probably not a dike; it’s too localized. It looks like a big blob of country rock that broke off and sank into the body of magma.
An outcrop to the south has clear rapakivi texture.
Rapakivi texture. 36
01.354N 106 50.945W
The weathering of this surface helped bring out the texture Here’s a sample.
The rapakivi texture refers to the big pink rounded crystals.
These are orthoclase with a rim of oligoclase, a sodium-rich
plagioclase feldspar. You can see the rim particularly well on the
big crystal towards the right side of the sample. The presence of
big, fat, and happy orthoclase crystals with reaction rims makes
this a rapakivi quartz monzonite; the oligoclase in the rims makes
it a particular kind of rapakivi called vyborgite. The bluish
grains of quartz are quite striking. The rock also contains dark
grains of biotite.
Though the presence of large orthoclase crystals is quite common in silica-rich intrusive rock, true rapakivi texture is fairly rare. It is usually associated with so-called A-type granite, of which we'll learn more shortly.
Further north, the monzonite shows a system of joints trending north to south, cutting across the shear zones noted earlier.
The joints are oriented nearly vertically.
The joints likely formed when this rock was at a shallower depth
than when the shear zones were formed. They may be related to the
most recent upthrust of this area, less than 25 million years ago.
Biotite is a mica with the formula KFe3AlSi3O10(OH)2.
It is common for magnesium to substitute for some of the iron and
fluorine to substitute for some of the hydroxyl. (Fluoride and
hydroxyl ions have the same charge and very nearly the same
diameter.) Biotite is very common in igneous rocks, being present
in everything from extrusive, low-silica basalt to intrusive,
high-silica granite.
Biotite differs from muscovite in having iron hydroxide (or
fluoride) rather than aluminum hydroxide act to bond pairs of
phyllosilicate sheets together. This modifies the structure
slightly. The ferrous hydroxide sheet does not consist of open
rings of cations bonded by hydroxyl, as with the aluminum
hydroxide sheet in muscovite, but of a dense sheet of packed iron
cations bonded by hydroxyl. Instead of two aluminum ions joining
each pair of rings of the two phyllosilicate sheets, three iron or
magnesium ions join each pair of rings. A mica having two metal
ions joining each pair of rings is described as dioctahedral,
while one having three metal ions join each pair of rings is
described as trioctahedral. The octahedral refers to a
site in the middle sheet surrounded by six oxygen or hydroxyl ions
where a metal ion of the right size could potentially be located.
Here's a sample of particularly coarsely crystallized biotite.
Biotite crystals in a sample of granite.
Like muscovite, biotite has a single perfect cleavage plane that allows the mineral to be split into very thin elastic sheets. However, biotite can usually be distinguished from muscovite by its very dark color and higher density.
Relief map of the Jemez with muscovite-biotite
outcroppings highlighted in red.
The San Pedro Peaks area is underlain by a pluton that appears to be younger than the quartz monzonite. This also crops out in the upper canyon of the Rio Puerco and Rito Resumidero.

Muscovite-biotite granite at Rito Resumidero falls. 35
35.946N 106 53.533W

Muscovitebiotite granite at Rito Resumidero falls. 35
35.946N 106 53.533W
This is described a muscovite biotite granite, containing
microcline and quartz and smaller quantities of sodium-rich
plagioclase and muscovite and biotite. Such two-mica granites
are thought to form primarily from aluminum-rich sedimentary beds
heated by basalt underplating. This granite has been dated as
1.700 billion years old.
The youngest sizable pluton in the San Pedro Mountains is thought
to be a mass of leucogranite, which is a light-colored
granite containing very little dark mafic minerals.

Leucogranite near Deer Mountain. 36.0162173N
106.7976472W

Leucogranite near Deer Mountain. 36.0162173N
106.7976472W
Leucogranites are usually interpreted as a product of the melting of thickened crust composed almost entirely of clay minerals.The leucogranite here is relatively fine grained as well as almost devoid of any iron minerals except traces of biotite. In some locations, it has bluish quartz and plagioclase grains thought to have melted out of the surrounding quartz monzonite.
With no radiometric date, we have almost no age constraints on
this pluton. We know only that it is younger than 1.73 billion
years and older than 25 million years, the age of a patch of
sedimentary rock overlying it to the north. But it is though to be
Precambrian, and I would hazard a wild guess it is around 1.4
billion years old, the age of similar granite intruded further
south that I'll describe shortly.
South of Señorita Canyon, the Sierra Nacimiento rises to maximum
elevations of 2424m (9264') at Big
Mountain, 2887m (9471') at San
Miguel Mountain and 2750m (9022') at Pajarito
Peak.

Pajarito Peak. Looking north from 35
35.946N 106 53.533W
The mountains here are less lushly forested than further north,
with pinon scrub dominating the lower slopes. Much of the
southernmost Sierra Nacimiento belongs to the Zia and Jemez
Pueblos, while most of the rest is National Forest.
Big Mountain is underlain by Phanerozoic rocks, but San Miguel
Mountain and Pajarito Peak are underlain by Precambrian rocks. The
Precambrian rocks of the southern Sierra Nacimiento Mountains
include some that are the right age and character to be assigned
to the Yavapai-Mazatzal transitional zone basement.
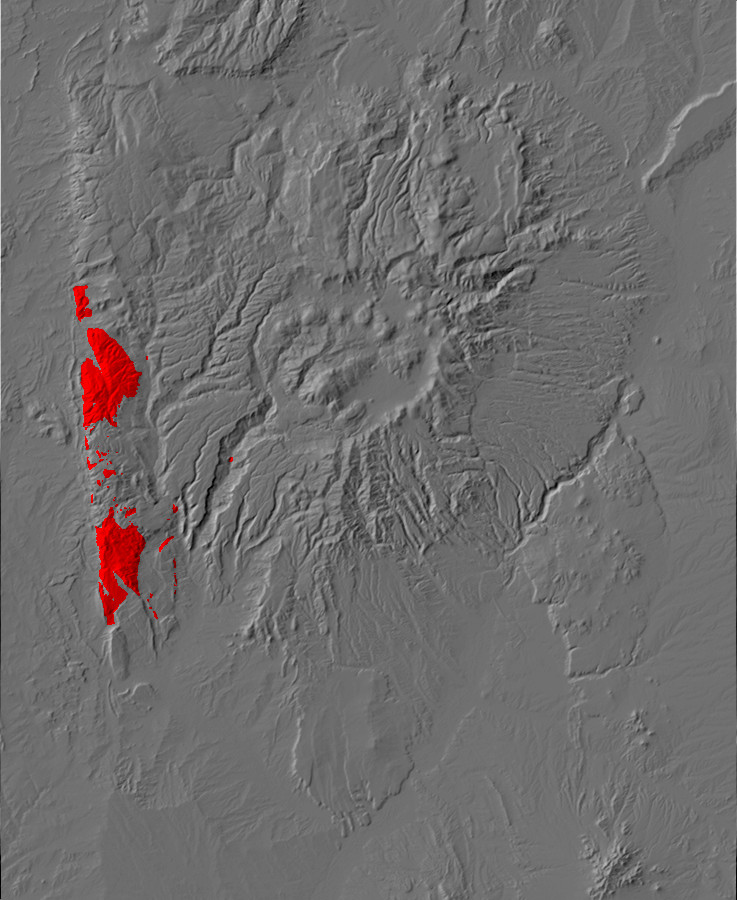
Relief map of the southern Jemez with Yavapai province
outcroppings highlighted in red.
The central Sierra Nacimiento is dominated by the San Miguel
Gneiss, which has a radiometric age of 1.695 billion years. This
is consistent with this rock being emplaced during the Yavapai
Orogeny.

San Miguel Gneiss. 35
50.677N 106 51.340W
The minerals here are granitic: biotite, quartz, and alkali
feldspar. The biotite forms distinct bands in the rock, leaving no
doubt it is a gneiss. Its composition is that of a monzogranite,
with more plagioclase than alkali feldspar and around 25 percent
quartz. Its age and character somewhat resemble the Tres Piedras
Granitic Orthogneiss, and it may have formed by the same process
along the suture zone.
Intrusive formations that have become exposed at the surface
sometimes retain beds of the overlying country rock that have not
quite eroded away. These are known as roof pendants and
take the form of localized outcroppings of rock that are different
in character from the rock forming the intrusion. They are also rootless;
that is, they do not connect with any deeper structure or nearby
outcroppings. There is a roof pendant in the San Miguel Gneiss
that caught the attention of geologists mapping the area. It is
just a couple of hundred feet across, is located on a heavily
forested hillside, and is not that well exposed. I have to admire
the geologists who were thorough enough in their mapping to
discover it.

Hornblendite outrcop in the San Miguel Gneiss.. 35
50.530N 106 51.110W
This rock is mostly hornblende, with just a scattering of
feldspar. This means a low silica content, perhaps as low as 45%.
Such rocks are described as ultramafic and they are fairly
uncommon. Its location in a crustal province originating in an
island arc suggests that this may be an ophiolite, a
fragment of oceanic crust produced in the Pilar back-arc basin and
trapped when the basic closed up.
The only Precambrian rocks exposed within the Jemez proper are
found in Cañon de San Diego
at Soda
Dam.
Cañon de San Diego is one of two natural highways into the Valles caldera and the only one that is open to the public today. (The highway routes from Cuba and Los Alamos are highly engineered.) Cañon de San Diego can be visited via State Road Four, which branches off U.S. 550, the Bernalillo – Farmington highway, at San Ysidro. The canyon is a geologist's playground, cutting through young volcanic beds of the Jemez to expose colorful Mesozoic and Paleozoic sedimentary beds of the eastern edge of the Colorado Plateau. The upper canyon is heavily forested with ponderosa pine, which gives way to pinon scrub forest in the lower canyon. Much of the lower canyon belongs to the Jemez Pueblo, while the upper canyon includes the village of Jemez Springs, several private developments, Hummingbird Music Camp, and numerous National Forest campgrounds. These make the canyon one of the more densely populated portions of the Jemez.
The ancestors of the Jemez Pueblo settled the area beginning around the 13th century, and some of their ruined pueblos can still be found in the canyon or atop the high mesas on either side. The pueblo of Giusewa was built at this time on the current site of Jemez Springs. The Spanish established a church beginning in 1621, San José de los Jémez, which can be visited today as part of the Jemez Historic Site.

San José de los Jémez. 35
46.700N 106 41.227W
The mission here served both Giuwesa and the pueblo at Vallecitos
de los Indios, the closest sizable settlement to the Valles
caldera. However, the church was abandoned by 1640, and the pueblo
was abandoned during the Pueblo Revolt of 1680 in favor of pueblos
in more defensible locations. The inhabitants never returned,
ultimately settling further down canyon where their descendants
live today.
San Diego Canyon roughly coincides with a major fault zone, the
Jemez Fault Zone, which is part of the western margin of the Rio
Grande Rift. At Soda Dam, the fault has brought up some of the
Precambrian basement rock. This is a granite gneiss with a
radiometric age of about 1.6 billion years.
View to the north from south of Soda Dam, which is partially visible as the low ridge just across the road. The Precambrian
gneiss towers over the road on the left.
35
47.481N 106 41.208W


As with the feldspathic schist from the Tusas, this rock is
mostly quartz and feldspar with some mafic minerals. The grains in
the rock are oriented in a common direction, which is not true of
an ordinary granite, whose crystals are oriented at random. This
indicates that the rock was once under great heat and pressure,
which caused it to recrystallize parallel to the shear forces it
was experiencing. In the case of the granite gneiss of the Soda
Dam area, the recrystallization is fairly subtle and the rock only
mildly metamorphosed, so that it superficially still resembles
fine-grained ordinary granite. Strongly metamorphosed granite
gneiss shows a characteristic banding that is not evident at Soda
Dam, but which we saw earlier with the San Miguel Gneiss. The lack
of schistose layering indicates this rock lacks mica and suggests
that it is not highly enriched in aluminum.
This relatively small outcropping of Precambrian gneiss has not been correlated with nearby Precambrian formations. The nearest Precambrian rocks that have been assigned formation names are in the Sierra Nacimiento Mountains just to the west. The rock here doesn't fully match the description of any of the granites further west, and it is older than any of these granites.
We've seen that the Precambrian rocks of nothern New Mexico include formations that are between 1.7 and 1.8 billion years old, typical of the Yavapai crustal province. These accreted to the southern edge of Laurentia during the Yavapai orogeny, 1.72 to 1.69 billion years ago, and this orogeny was accompanied by intrusion of plutonic rocks like those of the Maquinita Granodiorite, the Tres Piedras Orthogneiss, and the San Pedro Quartz Monzonite. There is a cover of metavolcanic and metasedimentary rocks of about this age, the Vadito and Hondo Groups, produced in a back-arc basin that accompanied this orogeny.
Geologist have long believed that the Mazaztal crustal province to the south accreted between 1.65 and 1.6 billion years ago, during an event called the Mazatzal Orogeny. Beds of Mazatzal age, 1.7 to 1.65 billion years old, are found in the Manzano Mountains south of the Jemez. Like the Yavapai crustal province, the Mazatzal crustal province is thought to have first formed offshore as island arcs, then accreted to the continent. Plutons emplaced during this orogeny may extend as far north as the southern Wyoming border.
The Mazatzal equivalent of the Moppin Complex includes the Tijeras Greenschist, found in Tijeras Canyon east of Albuquerque.

Tijeras
Greenschist. 35.0803682N
106.4031864W
This has been dated as 1.659 billion years old, typical of the
Mazatzal Province. No rock south of the Jemez Lineament in New
Mexico has been dated as older than 1.7 billion years. The
Sevilleta Metarhyolite resembles the Vadito Group rhyolites but is
younger at 1.67 billion years. Plutons intruding these rocks have
ages clustered tightly around 1.66 billion years. One such pluton
is the Cebola Gneiss.

Cebola Gneiss. 35.0669444N
106.4332057W
Just to the west is spectacular evidence of the violence with which the Mazatzal Orogeny jammed the island arc against the continental margin.

Sheared granite.
35.0669444N
106.4332057W
The rock here is a mix of intact granite and granite that has been heavily deformed.

Sheared granite.
35.0669444N
106.4332057W
This is the northern edge of the Manzano Thrust Belt, or at least
the northern end of what's still preserved. The rocks at this spot
have been sheared past each other by the force of the collision of
the Mazatzal island arc with the continental margin of the Yavapai
province. This is known as the Seven Springs shear zone, and there
are at least three other shear zones paralleling it to the south
in the northern Manzano Mountains.
This orogeny jammed the Jemez subduction zone to create the Jemez
Lineament. It also deformed the crust northward into the Yavapai
Province, resetting some radiometric clocks and partially
obscuring the boundary between the provinces to help form the
Yavapai-Mazatzal transition zone. With this understanding, the
Yavapai-Mazatzal transition zone properly belongs to the Yavapai
province. Some of the younger plutons of the southern Sierra
Nacimiento Mountains carry the isotopic signature of lower crust
that belongs to the Yavapai Province, placing the boundary with
the Mazatzal Province south of the Sierra Nacimiento at the
southernmost edge of the Jemez Lineament.
The period between 1.8 and 0.8 billion years ago, from the late
Paleoproterozoic to the mid-Neoproterozoic, has been dubbed the
"Boring Billion" by some geologists. This was a long period of
relative tectonic stability, in which the atmospheric oxygen level
held fairly steady at less than 10% of its current value, and in
which life evolved only slowly. However, this time period was not
completely uneventful. For one thing, by 1.4 billion years ago,
the supercontinent of Columbia was showing signs of breaking up,
only to reassemble within 200 million years into the
supercontinent Rodinia. And something was cooking under what is
now western North America.
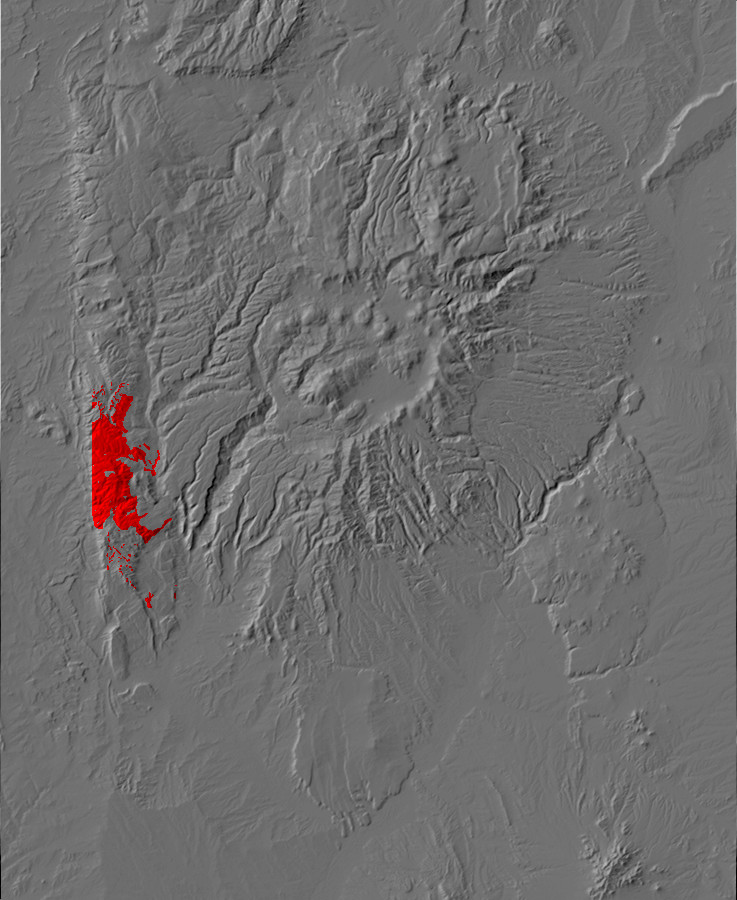
Relief map of the Jemez with Joaquin Granite
outcroppings highlighted in red.
Following the end of the Mazatzal Orogeny 1.65 billion years ago, the Jemez region experienced a period of tectonic quiescence. This ended around 1.45 billion years ago, when the Precambrian rocks of the Mazatzal and Yavapai Provinces were intruded in many locations by huge granite or granite-like bodies, called batholiths. The 1.45 billion year batholiths are found across the western United States, constituting fully 15% to 40% of the Precambrian surface. These batholiths point to a major episode of widespread crustal heating whose cause was long hotly debated by geologists.
Granite from one such batholith can be found in the Rio Guadalupe
Canyon, at the mouth of the tributary Joaquin Canyon.



and throughout the southern Sierra Nacimiento.

Joaquin Granite. 35
49.459N 106 50.160W
This is the Joaquin quartz monzonite which is the most common Precambrian rock in the southern Sierra Nacimientos. It's a true granite with a radiometric age of 1.424 billion years.
There is a beautiful and easily accessible outcropping of Precambrian quartz monzonite at the Guadalupe Box.

Southern entrance to Guadalupe Box. 35
43.876N 106 45.687W
The road here passes through a pair of tunnels, the Gilman
Tunnels, which were blasted out the rock in the 1920s to make way
for the Santa Fe North Western Railroad. By the time the tunnels
were completed, in August 1924, they had accounted for half the
cost of construction of the railroad. Timber was loaded onto the
rail cars at Deer
Creek Landing and ruins of logging camps can still be found
on Peggy
Mesa, west of the tunnels. The tunnels and settlement were
named for William H. Gilman, the vice president of operations of
the railroad.
The Great Depression and a series of railroad accidents brought
logging to a near halt by 1937, but logging resumed under the New
Mexico Timber Company in the 1940s, which constructed the town of
Gilman,
Today this is a small cluster of homes with a craft shop. The
company also removed the rails to make way for trucks, which were
both cheaper and safer to operate. Logging again came to a near
halt in the 1960s and the tunnels were deeded to the Forest
Service, which renovated the bridges approaching the tunnels and
paved the road for automobiles.
The quartz monzonite itself is not much to look at when weathered.

But fresh surfaces reveal just how gorgeous a stone this is.


Strictly speaking, this beautiful rock is a hornblende-biotite
quartz monzonitic gneiss. The large feldspar crystals are more
typical of high-silica intrusive rocks than the rapakivi texture
of the San Pedro Quartz Monzonite: They are not as rounded and do
not show an oligoclase rim. The quartz is less striking and this
rock is more abundant in iron-rich minerals. It has also been
metamorphosed, though the foliation from metamorphism is barely
evident here.
The monzonitic gneiss of Guadalupe Box is 1.450 billion years in
age. Its isotope makeup indicates it was extracted from lower
crust of Yavapai age. This has forced geologists to revise their
estimate of where the Yavapai-Mazatzal boundary is located at the
longitude of the Sierra Nacimiento Mountains. Rather than running
along the Nacimiento Creek shear zone, as previously thought, it
appears to run south of the Nacimiento Mountains.
Across the Rio Grande Rift from the Sierra Nacimientos and the Jemez Mountains is the southern Sangre de Cristo Mountains. This area is also thought to be part of the Yavapai Province, and the rocks are a confusion of 1.6 to 1.7 billion year old biotite schist and granite gneiss intruded by 1.4 billion year old granite of the Picuris orogeny.

Further up the road, the entire assemblage is distorted from intense metamorphism.

The biotite schist is typical of metamorphosed oceanic crust,
and is evidence that much of the Mazatzal Province to which it
belongs came from ancient island arcs that accreted onto the
southern coast of Laurentia.
Sandia Crest is underlain by a batholith with an age of about 1.44 billion years.


Sandia Granite. 35.0645324N
106.4520037W
The black inclusion is regarded as evidence that the granite was
produced by basalt underplating. Similar mafic inclusions are
fairly common in the granite.
The 1.4-billion-year-old batholiths found throughout the Yavapai
and Mazatzal Provinces were long thought to be anorogenic,
meaning that they did not appear to have been associated with a
major episode of mountain building. The granites making up the
batholiths were described in turn as A-type granites, with the A
standing for anorogenic; but it can also stand for alkaline,
without any judgment on its mode of origin. This turns out to have
been an excellent hedging of bets. The A-type granites have a
distinctive chemical composition, being rich in silica and
alkaline metals (sodium and potassium) and having a high ratio of
iron to magnesium and a low calcium content. However, the
discovery that some of the metasedimentary formations in the
Picuris Mountains were much younger than previously thought, and
have roughly the same age as the "anorogenic" batholiths, provides
strong evidence that an orogeny did take place at this time.
One of the formations that suggests that there was a Picuris Orogeny 1.4 billion years ago is the Pilar Formation.
The Pilar Formation was long thought to have been laid down just
after the 1.7-billion-year-old Rinconada Formation. Radiometric
dating of a metamorphosed tuff bed in the formation finds that it
is much younger than originally thought, with an age of 1.488
billion years.
Pilar Phyllite. 36.211153N
105.8366517W

Pilar Phyllite. 36
12.661N 105 49.761W
This rock is particularly interesting because of its high content of graphite, which is thoroughly disseminated through the quartz and muscovite making up the bulk of the rock. It is unlikely we are looking at abiogenic carbon in this rock, which therefore shows the earliest signature of life in northern New Mexico. This rock was probably laid down in a shallow basin, rich with nutrients from nearby volcanic activity, in which cyanobacteria thrived and extracted carbon from the atmosphere.
The upper part of the formation contains beds of volcanic ash
that have been metamorphosed to mica schist.

Pilar Phyllite. 36.2123043N
105.8319887W
The ash contained zircons, and these have survived metamorphosis
with their load of radiogenic lead intact, giving us the
radiometric age of 1.488 billion years for this formation.
The revised age of this formation, and of the overlying Piedra
Lumbre Formation, show that highlands were raised 1.4 billion
years ago, the Pilar Basin was reactivated, and new sediments
accumulated in the basic. This establishes that the batholiths of
1.4 billion years ago were not anorogenic after all.
The Pilar and Piedra Lumbre Formations have been assigned to the new Trampas Group.
This formation lies immediately above the Pilar Formation.

Contact of Pilar Formation
with Piedra Lumbre Formation. 36.209398N
105.8280428W
The contact is the white zone at center. At right are thin beds of the Pilar Formation; at left is the Piedra Lumbre Formation. It is more massively bedded and includes some thick quartzite.

Piedra Lumbre Formation. 36.209398N
105.8280428W
This formation has only a maximum age, based on zircons within the formation and the age of the Pilar Formation below, but this maximum age is essentially the age of the Pilar Formation: About 1.45 billion years old.
The formation is separated from the next formation, the Marquenas
Formation, by the Plomo fault.

Plomo fault. 36.209398N
105.8280428W
The relatively flat rock face here is the plane of the fault. The
more poorly exposed rock to right is the Marquenas Formation.
Because the Piedra Lumbre and Marquenas Formations are in contact only across the fault, the age relationship cannot be determined stratigraphically, but must be inferred from other evidence.
The Marquenas Formation is mostly a rather dirty quartzite

Marquenas Formation with small pegmatite dike. 36.208254N
105.8273751W
Here's a sample.

The dark layer in this sample contains a small amount of magnetite, which imparts the dark color. When a piece from this layer is crushed, it is found to be mostly colorless quartz grains with a small percentage of much smaller black magnetite grains, which can be separated out with a kitchen magnet.
The lowermost beds of the Marquenas Formation include some beautiful metaconglomerates visible along the main highway through the Picuris Mountains.

Metaconglomerate of the Marquenas
Formation. Picuris Mountains. 36
12.220N 105 48.424W
The outcropping is quite extensive and is striking in appearance, looking like a dry river bed that has been spray painted with gold paint.
Metaconglomerate of the Marquenas Formation
The clasts are quite large, well-sorted, and well-rounded. The conglomerate was subsequently deeply buried and subjected to metamorphosis, to the point that the clasts have been deformed so that they are all flattened in the same direction. The luster is probably from sericite, which is a particular fine-grained form of muscovite mica, KAl2(AlSi3O10)(OH)2.
Here's another example.
Conglomeratic lower section of Marquenas Formation. Near 36.2041322N 105.8092104W
This is described as a highly strained conglomerate. The clasts
are all rather flattened and aligned in the same direction. This
is not thought to be the nature of the original sediments, but the
result of severe compression that deformed the original rounder
clasts.
The middle section of the formation is a fairly uniform quartzite.
Middle section of Marquenas Formation. Near 36.2047462N 105.8099698W

Marquenas Formation quartzite sample
Outcrops like this one preserve enough sedimentary structure to
allow geologists to determine which way the beds originally were
oriented. They find that the beds are actually overturned here,
with the younger beds to the north (lower left in the photograph.)
The uppermost beds are a pebble to cobble conglomerate.

Marquenas Formation upper conglomerate.
36.2078873N
105.8111237W
The Marquenas Formation was long thought to be part of the Vadito Group, perhaps correlating with the Big Rock Conglomerate in the Tusas Mountains. It has similar characteristics and it seemed to be in the right stratigraphic position. Its contact with the lower Vadito Group is exposed along the highway east of Dixon.

Contact of Vadito Group with Marquenas Formnation. 36.2036184N
105.8123434W
The contact is almost parallel with the face of the road cut. The
flat, nearly featureless beds at left are Vadito Group schist; the
rock beneath, exposed to the right, is basal conglomerate of the
Marquenas Formation. It looks like the one set of beds are a mere
continuation of the other.
However, in 2011, a team of geologists reported on detrital
zircon dating that demonstrated that the Marquenas Quartzite was
much younger than the Vadito Group. They found that most of the
zircons from the Vadito beds were from 1.765 to 1.704 billion
years old, and none were significantly younger. This demonstrated
that these beds were formed from sediments that were mostly eroded
off newly raised highlands of the Yavapai orogeny, with a small
contribution from older highlands further north. The Marquenas
Quartzite yielded large numbers of zircons that were as young as
1.435 Mya billion years old. The quartzite cannot be older than
the youngest zircons it contains, so the contact in the photograph
records a gap in the geologic record of 270 million years. The age
of the Marquenas Formation provides yet more evidence of a Pilar
Orogeny 1.4 billion years ago.
Iron readily contributes two electrons to the chemical compounds it forms, and iron in this state is known as ferrous iron to geologists. Chemists speak of such iron as having an oxidation number of +2, which is also the charge of a ferrous ion. With a little coaxing, the ferrous ion can contribute a third electron as well, forming ferric iron, with an oxidation number of +3. Both are found in the earth's crust today, but ferrous iron predominated in the early Earth, before cyanobacteria began generating oxygen. Because oxygen is scarce even today in the depths where magma is generated, ferrous iron is significantly more abundant in most igneous rocks than ferric iron.
Magnetite is a bit of a funny critter. Ferrous oxide has the
composition FeO, since the two electrons donated by each iron ion
match the two electrons needed by each oxygen ion. Ferric oxide
has the composition Fe2O3, reflecting the
additional oxygen needed to accept the third electron from each
iron ion. Magnetite has the composition Fe3O4,
suggesting that the iron in magnetite is in a kind of halfway
state, with an average oxidation number of +2.5. One can think of
magnetite as having the composition (FeO)(Fe2O3).
The evidence from crystallography is that there really are
separate ferrous and ferric ions in the crystal lattice, each
occupying their own pattern of sites, and by a quirk of chemistry,
this structure is unusually stable.
Here is a nest of large magnetite crystals.

Nest of large magnetite crystals from Bolivia.
Here's a large single crystal of magnetite.

Large single crystal of magnetite from western
Australia.
Individual large crystals like this are uncommon enough to be valued by collectors. The crystal is octahedral, opaque, and strongly magnetic, easily picked up with a kitchen magnet in spite of its fairly high density.
The magnetite in the Marquenas Quartzite is probably a placer deposit, formed in a stream bed or along a beach, where heavy, inert minerals, such as magnetite, tend to be concentrated. Here's a photograph of a modern magnetite placer in the Jemez. This is on a much smaller scale than the placers preserved in the Marquenas Quartzite, but it gives the idea.

Modern magnetite placer. Near 35.734N
106.618W
Placer deposits are of considerable economic importance, because precious metals and valuable metal ores are heavy and tend to be concentrated in them. Many of the most important gold deposits are placer deposits, such as this one near Fairplay, Colorado.

Gold placer mine Near 39
13.614N 106 0.414W
The piles of rubble visible here are tailings from placer mining. The river gravels here are rich in gold from veins in the mountains further up the valley and have been exploited for over a century.
The amateur prospector panning for gold is exploiting a placer deposit. Other precious and rare earth metals, tin, and diamonds are also extracted from placer deposits in various parts of the world.
The Picuris Orogeny was violent enough to produce considerable faulting and folding of the rock beds in the orogenic belt. Some rock beds broke and were thrust over older beds. Other rock beds, at greater depth or composed of softer sediments, were ductile enough to be folded instead. This produced structures such as the Copper Hill anticline.

Copper Hill anticline. Looking north from 36.208422N
105.8116617W
The terrain on which I am standing is Marquenas Quartzite. Just
out of site in the wooded area in the foreground is the Plomo
Fault, where the Marquenas Quartzite was thrust over the
underlying Piedra Lumbre Formation. The dark hills beyond
are Pilar Formation, and the white terrain across the most distant
drainage is Rinconada Formation. This is capped by a bed of Ortega
Formation forming the upper surface of the hill. The whole
structure is warped upwards along the hill, which is therefore an
anticline. Notice that the oldest beds are on top: The
structure is part of a fold and the entire sequence of beds has
been inverted.
Out of sight beyond the hill is the Hondo syncline, where the
beds are warped downwards. Here the folding has put the beds in
the normal order, youngest on top, with the Pilar Formation
filling the center of the syncline.
The Picurus orogeny was quite violent. Not only did it reactivate
the Pilar basin; it had the effect of converting the mafic crust
that originally assembled into the Yavapai and Mazatzal Provinces
to mature crust. The average composition of the crust changed from
that of basalt to that of andesite, as the more mafic material
settled to the base of the crust and delaminated into the mantle.
Yet we remain uncertain of just what it was that collided with the
southern margin of Laurentia 1.4 billion years ago. One school of
though is that a previously unrecognized crustal province, the
Granite-Rhyolite crustal province, collided with Laurentia during
the Picuris orogeny. This crustal province is poorly exposed but
seems to have an age of 1.55-1.35 billion years. Another school of
thought is that there wasn't a distinct Mazatzal Orogeny,
and the Picuris Orogeny is when the Mazatzal crustal province was
finally sutured to Laurentia. However, the Sandia, Manzano, and
Los Pinos mountains south of the Jemez Lineament, part of the
Mazatzal Province, show clear indications of a pulse of
simultaneous deformation and pluton emplacement 1.65 to 1.66
billion years ago that points to a distinct Mazatzal Orogeny.
Further complicating our picture is the evidence that Colombia began breaking up at about the time of the Picuris Orogeny. This suggests that accretion continued along southern Laurentia even as Antarctica and Australia were rifting away from western Laurentia. Possible evidence for this is the presence of numerous dikes of this age in the Jemez region.
In the Tusas Mountains, rock of 1.4 billion years in age mostly
takes the form of dikes. For example, dikes of almost pure quartz
cut across the Moppin Complex on Hopewell Ridge.

Large quartz vein. Near 36
38.339N 106 07.740W
Dikes form when magma forces its way through a fissure in the
country rock and then cools in place. They are perhaps the most
common form of intrusive body or pluton.
An impressive pair of pegmatite dikes are found at a road cut in the southernmost Tusas Mountains.

These dikes are located just a few yards from each other along the same road cut. It is quite common to see multiple dikes running parallel with each other. Where a large number of parallel dikes are found in a region, geologists often refer to them as a dike swarm. There are numerous pegmatite dikes in the Tusas Mountains, though probably not so many that they constitute a dike swarm.
Notice that the foliation in the country rock (of the Burned
Mountain Formation) appears to be present in the pegmatite as
well. This suggests that there was a significant episode of
metamorphic deformation following the emplacement of the
pegmatites.
Pegmatites are notable for the presence of very coarse crystals,
sometimes of quite unusual minerals.
This pegmatite is full of large crystals of quartz, feldspar, muscovite, and accessory minerals. Such large crystals do not form from slow cooling alone. The magma from which they form must also be rich in water vapor, which greatly lowers its viscosity. This water vapor also accounts for the presence in pegmatites of minerals such as mica that include water in their crystal structure.
Pegmatites are thought to form from the very last part of a granitic magma chamber to crystallize, and so they tend to contain unusual minerals containing incompatible elements. Incompatible elements are elements having a combination of ionic radius and electrical charge that is significantly different from those of the more common rock-forming elements. As a result, these elements are reluctant to enter ordinary rock-forming minerals as a trace constituent. For example, manganese is nearly identical to iron in its charge and ionic radius, making it a compatible element, and it commonly substitutes for iron in iron-bearing minerals. This is why distinctive manganese minerals are not terribly common, even though manganese is a fairly abundant element. Boron, on the other hand, has a charge and radius unlike the more common elements, making it an incompatible element. Though a very rare element, it tends to concentrate in the residual magma fluids that form pegmatites, which therefore often contain tourmaline or other distinctive boron minerals. Other incompatible elements found in pegmatites include lithium, beryllium, fluorine, tin, niobium, tantalum, and certain lanthanide metals. The unusual composition makes pegmatites attractive to prospectors, and there are many old mines in the Tusas Mountains.
One such mine is the Joseph Mine, located just a couple of miles north of the small resort of Ojo Caliente. We saw a panorama of the hilly terrain north and west of the resort earlier in this chapter. This terrain is underlain mostly by Vadito Group metarhyolite, amphibolite, and schist, intruded in numerous locations by pegmatite dikes. The Joseph Mine itself is located in a large pegmatite plug that has intruded the boundary between metarhyolite and amphibolite outcrops. The mine takes the form of a sizable open pit.
Joseph Mine. 36
19.646N 106 03.324W
The A-type pegmatites of the Tusas Mountains are rich in aluminum, and the combination of high aluminum and potassium content is favorable for forming muscovite mica. Mica has been extensively mined from the Tusas Mountains and was the principal product of the Joseph Mine. Some of the mica here was truly spectacular, forming “books” (individual crystals) exceeding three feet in diameter. Even today, it is easy to find mica books six inches across.
Mica books at Joseph Mine. Car keys at
lower right for scale.
More such books are exposed in the short adits (horizontal tunnels) cut into the pegmatite nearby. These are difficult to extract intact, but I managed the following specimen.
Muscovite from Joseph Mine
Unfortunately, this fell apart before I could wrap it up. But I managed to get some other large specimens home intact.
Muscovite from Joseph Mine
It’s not obvious in this photograph, but this sample is nearly
five centimeters (two inches) thick. It's a single crystal of
muscovite.
Amphibolite that is intruded by pegmatite often contains almandine garnet near the contact. Individual garnet crystals can be found weathered out of the contact between the pegmatite and the adjoining amphibolite of the Joseph Mine. Few of these are gem quality, but they can still be fun to hunt down and collect. As with fossil hunting, you have to train your eye to spot garnets mingled with the pebbles along the slopes below the amphibolite.
Garnets from Joseph Mine
The crystals are imperfect, but you can see crystal faces on the
better samples.
Garnet is an example of a nesosilicate, in which isolated
silica tetrahedra are completely surrounded by metal ions that
provide charge balance. The composition of garnet is highly
variable; almandine typically has the composition Fe3Al2(SiO4)3,
but almost any metal ion with a charge of +2 can substitute for
the iron and either ferric iron or chromium can substitute for the
aluminum.
Garnet is found almost exclusively in high grade aluminum-rich
metamorphic rock. At the Joseph Mine, the metamorphism was caused
by a nearby hot body of magma (the pegmatite), a process which is
called contact metamorphism. This is in contrast with the
regional metamorphism that produced the extensive
metamorphic formations of the Tusas and Picuris Mountains, which
was caused by an orogeny.
Staurolite beds almost always contain garnets as well. The
reverse is not true: Most garnet-bearing rock does not contain
staurolite. Garnets do not require as unusually high an aluminum
content as staurolite, and they are stable over a much broader
range of pressures and temperatures, so that garnet is a fairly
common mineral in aluminum-rich metamorphic rock.
One of the unusual minerals found at the Joseph Mine is tourmaline. This is found mostly on the western rim of the mine, close to the contact of the pegmatite with the host amphibolite.
Tourmaline is a cyclosilicate mineral, whose basic framework is stacked rings of silica tetrahedra. These are bonded to triangular borate ions by various metal ions. The type of tourmaline found at Joseph Mine is schorl, in which the metal ions are predominantly iron and sodium, yielding a formula NaFe3Al6(BO3)3Si6O18(OH)4. You can see that the schorl takes the form of long black striated rods. A wide range of metal ions can substitute for sodium, iron, aluminum, and silicon, making tourmaline one of the minerals most variable in composition. The sodium and borate come from the pegmatite, while the iron comes from the amphibolite, and silica and aluminum comes from both. Alteration in the composition of country rock by fluids from an intrusion is called metasomatism.
Along with muscovite and accessory minerals like garnet and
tourmaline, the pegmatite at Joseph Mine is composed of quartz and
alkali feldspar. These are visible in this outcrop.
The reddish mineral is probably microcline, while the white could
be either albite or quartz. There is also considerable muscovite.
I picked up a nice sample of feldspar here.
This is a cleavage fragment from a single large crystal. One can hold the sample to the light and see that the entire surface reflects the light at the same angle. The fine striations suggest that this is perthite, composed of thin alternating layers of albite and microcline, which separate from each other as the feldspar slowly cools.
The Harding mine exploits a pegmatite body rich in unusual minerals containing rare elements. This body was formed around 1.3 billion years ago, towards the end of the Picuris orogeny. Like the Joseph Mine, the Harding Pegmatite Mine is mostly quartz, alkali feldspar, and mica. However, it is a complex pegmatite rich in other valuable elements. It was originally mined for lithium, which was used in Pyrex glassware. Some of the minerals found at the mine contain upwards of 6% lithium oxide, which is vastly enriched over the average value in the earth’s crust (less than 70 parts per million.) This did not long remain profitable and the mine closed in 1930. However, during the Second World War, the mine was prospected again and found to have significant amounts of tantalum, a metal used to produce steel alloys that are highly resistant to corrosion. Mining resumed in 1942, and, for a time, the Harding mine supplied most of the free world’s tantalum. Production shifted to beryllium in 1950, but mining ceased for good in 1958, and the mine was later donated to the University of New Mexico for its educational value. The mine now has signage and numbered stations for a walking tour; visits must be arranged in advance through UNM.
This is how the pegmatite exposure likely looked when it was
first discovered. Similar outcroppings extend some distance east
along the contact between the amphibolite and the mica schist,
Unexploited pegmatite near Harding pegmatite mine. 36.1926746N 105.7937405W
The exposed surface is mostly large weathered outcrops of quartz, which prospectors associated with possible valuable ore depots. Also visible near this location is clevelandite.

This is a form of albite with peculiar curved platy crystals. It
tends to cut across other zones in the pegmatite, but is more
typical of the center of the intrusion
Like the Joseph Mine, the Harding Pegmatite Mine intrudes between
amphibolite and schist facies of the Vadito Group, and it has some
well-crystallized tourmaline in the contact with the amphibolite.
Because the pegmatite cooled and crystallized so slowly, it is
a zoned pegmatite,
with certain minerals found right at the contact with the
surrounding rock, a layer with slightly different composition away
from the contact, and yet other layers deeper in the pegmatite.
This is the margin of the pegmatite.

This boulder preserves part of the contact of the pegmatite with
the overlying very dark amphibolite. You can see a very
definite chilled margin where
the pegmatite cooled relatively quickly in contact with the
amphibolite to produce fairly fine-grained rock. This is likely
mostly quartz and feldspar. Next to the chilled margin is a zone
variously described as the berry zone or the beryl zone. Apatite,
tantalite, and beryl are found in this zone, the latter sometimes
as huge crystals.
The spodumene zone lies between the beryl zone and the spotted rock.
Spodume zone of Harding
pegmatite mine. 36.1925343N
105.7953866W
The large criss-crossing lathes are crystals of spodumene, a lithium pyroxene mineral that was one of the richest lithium ores mined here. Some of the crystals are enormous.
Spodume from Harding pegmatite mine. 36.1925343N 105.7953866W
This zone also has lovely lavender outcrops of lepidolite.
Lepidolite from Harding pegmatite mine. 36.1925343N 105.7953866W
There’s probably not that sharp a distinction between lepidolite and lithium-rich rose muscovite. The tendency seems to be to label the fine-grained, lavender stuff as “lepidolite” and the coarser, ruddier stuff, like this
Rose muscovite from Harding pegmatite mine. 36.1925343N 105.7953866W
as rose muscovite. The spotted rock at the core of the intrusion is quite distinctive.
Spotted rock with rim of berry zone at Harding pegmatite mine. 36.192783N 105.7954862W
This is probably the best spot to see the zoning. The top of the pinnacle was the upper surface of the dike, from which the amphibolite has been eroded. The gray cap is the berry zone. The lighter zone below and curving around to the left is the quartz zone. There is little left of the quartz-spodumene zone except a region of more abundant mica in the upper part of the spotted rock.
There are crystals of apatite in the berry zone atop the pinnacle.
Apatite at Harding pegmatite
mine. 36.192783N
105.7954862W Public domain
MIcrolite, a tantalum-niobium
ore, was another important mine product.
The microlite is the yellowish-brown patches.
Those wishing to visit the Hardingn Pegmatite Mine should contact the Department of Earth and Planetary Sciences at UNM.
Pegmatite dikes are also found in the San Miguel Gneiss. This
example is poorly exposed, but gives some idea.

Pegmatite in the San Miguel Gneiss.. 35
50.581N 106 51.543W
This pegmatite is rich in quartz with some feldspar, but little
of any other kind of mineral. Pegmatites that contain few
accessory minerals are known as simple pegmatites. It
might also be classified as a leucogranite. Some quite sizable
leucogranite outcrops occur in the southern Sierra Nacimientos.
Nearby is another dike of very different character.

Mafic outcrop in the San Miguel Gneiss.. 35
50.574N 106 51.527W
This appears to be an ultramafic intrusion of some kind. Under
the loupe, it looks a little like the hornblendite from earlier in
this chapter, but with only the barest scattering of feldspar and
with some significant content of biotite. My geologic map for this
area notes that there are localized outcrops of schistose
amphibolite in the San Miguel Gneiss, though it does not
specifically show this one on the map. But that's probably what
this is. Was this a mafic dike, since heavily metamorphosed, or
another bit of ocean crust trapped when the Mazatzal and Yavapai
Provinces were sutured together? And is there any significance to
its location right next to a pegmatite dike?
While it is a common view that pegmatites form from the last
fraction of a magma to solidify, it is also possible that some
pegmatites form from the reverse process, where regional
metamorphism heats rock in the middle crust just enough for the
rock to begin to melt. This melt will be rich in volatiles and
incompatible elements, and if it then moves from its source region
to a higher level of the crust, it could produce a pegmatite
difficult to distinguish from one formed from the last liquid
fraction of a large magma body. The chief difficulty with this
theory is explaining how the small amount of melt separates from
its source rock. Melted rock is expected to cling to the remaining
solid rock like water in a sponge. None can be extracted until the
source rock has melted enough for its pore spaces to be saturated
with magma.
Dikes, particularly dike swarms, are associated with crustal extension. The large number of dikes in the Jemez from 1.4 billion years ago suggest the area experienced such extension during the breakup of Columbia. However, the dikes are mostly not mafic in composition, as is typical of dikes associated with rifting. Nor could silica-rich rock making up the Picuris-aged batholiths of the western United States have formed directly from silica-poor magma produced in the mantle. The primitive magma must have risen to the base of the crust because it was less dense than the upper mantle, then spread out laterally because it was more dense than the overlying crust. Only small quantities of this magma reached the surface as mafic dikes. This magma was very hot, and it provided both a source of water vapor and heat to melt the more silica-rich rock above it. This rock was already rich in water-containing minerals from its island arc origin and so was fertile for magma production. This magma further differentiated, leaving silica-poor minerals at the base of the crust while continuing to ascend to become part of a more silica-rich upper crust. This zoning of the crust is typical of continental crust throughout the world today. It is possible that delamination subsequently removed the residual iron- and magnesium-rich rock at the base of the crust, increasing the buoyancy of the crust even further.
A common characteristic of the 1.45 billion year plutons and the
associated pegmatites is that they look almost pristine. There is
some jointing in these rocks where they crop out, but there is
practically no discernible foliation or other signs of deformation
in these intrusions. Nothing comparable to the Yavapai, Mazazatl,
or Picuris Orogenies has affected northern New Mexico in the last
1.4 billion years. Even the birth of the Ancestral Rockies, which
we'll learn about shortly, and the rise of the modern Rocky
Mountains has not been violent enough to affect the basement rocks
to any comparable degree.
The following animation illustrates the formation of the crust under New Mexico. It is based on radometric dates for Precambrian rocks in the region, with a dot appearing at the location from which each sample was taken at the time corresponding to its radiometric age. This shows the emplacement of Precambrian rocks from 1800 million years ago to 1110 million years ago. Note how rocks are first formed in southern Colorado and northern New Mexico (Yavapai province) then activity abruptly spreads south (Mazatzal provice.) There is a long period of quiescence, then the anorogenic pulse of magmatism takes place at about 1.4 billion years. There is a final small burst of activity in southernmost New Mexico corresponding to the assembly of Rodinia at around 1.1 billion years.
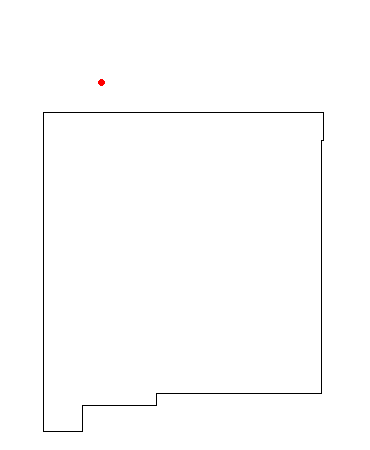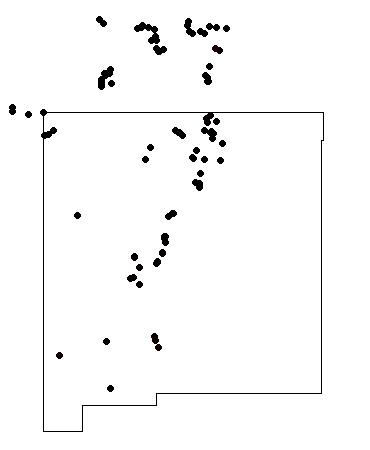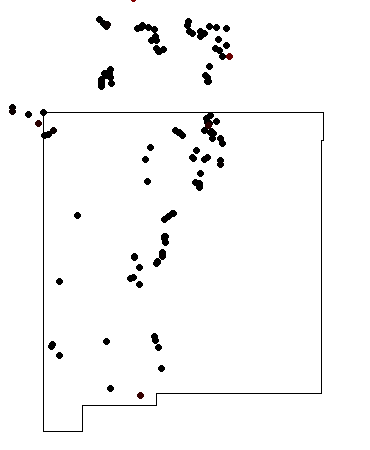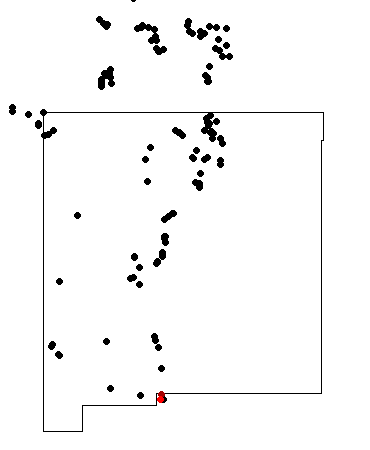
With all these pieces of the puzzle in place, we can finish our story of the Jemez in the Precambrian, acknowledging that there are still long blank periods and that there will doubtless be future retellings.
1.71 billion years ago, an island arc that had formed in the
ocean west of Laurentia drifted into the western edge of the
continent (which would be south in the modern orientation.) The
collision twisted and deformed the great stacks of basalt and
andesite underlying the arc, which would someday be exhumed as the
Moppin Complex, the Maquinita Granodiorite, and the San Pedro
Quartz Monzonite. The force of the collision threw up mountains
and emplaced fresh bodies of magma that would someday be exposed
as the San Miguel Gneiss and the Tres Piedras Orthogneiss.
A line of volcanoes formed along the south edge of the newly accreted block, and a back arc basin formed behind the mountain front. The crust was thinned enough to permit fresh basalt to erupt into the basin. This was overlain by volcanic ash erupted from great volcanoes along the arc. These would someday become the Vadito Group.
Erosion in the rugged highlands produced great quantities of sand that were deposited in the basin atop the earlier volcanic beds. This would someday be the Ortega Formation. A river system developed north of the basin and deposited delta sediments atop the sand that would become the Rinconada Formation.
Thirty million years later, another island arc drifted into the
continent, jamming the subduction zone and shifting subduction
further south. Remants of the original subducting slab would
become the Jemez Lineament. The newly accreted island arc became
the Mazatzal Province.
Two hundred million years passed in relative tranquility.
1.45 billion years ago, a large microcontinent drifted into the
continental margin. The accretion of the Granite and Rhyolite
Provinces was a violent affair, similar to the geologically much
more recent accretion of the Indian subcontinent onto south Asia.
The crust was deformed for a thousand kilometers (six hundred
miles) inland from the coast, creating a mountainous plateau
similar to Tibet. Great bodies of magma were intruded into the
deformed crust, transforming it from juvenile crust into mature
continental crust. Meanwhile, fresh sediments were deposited atop
the now 200-million-year-old Rinconada Formation that would
someday be the Trampas Group.
There followed almost a thousand million years of quiescence,
which belong to the next chapter of our story.
Next chapter: When the Jemez
was beachfront property.
Copyright ©2014-2018 Kent G. Budge. All rights reserved.